Figure 1
Download original image
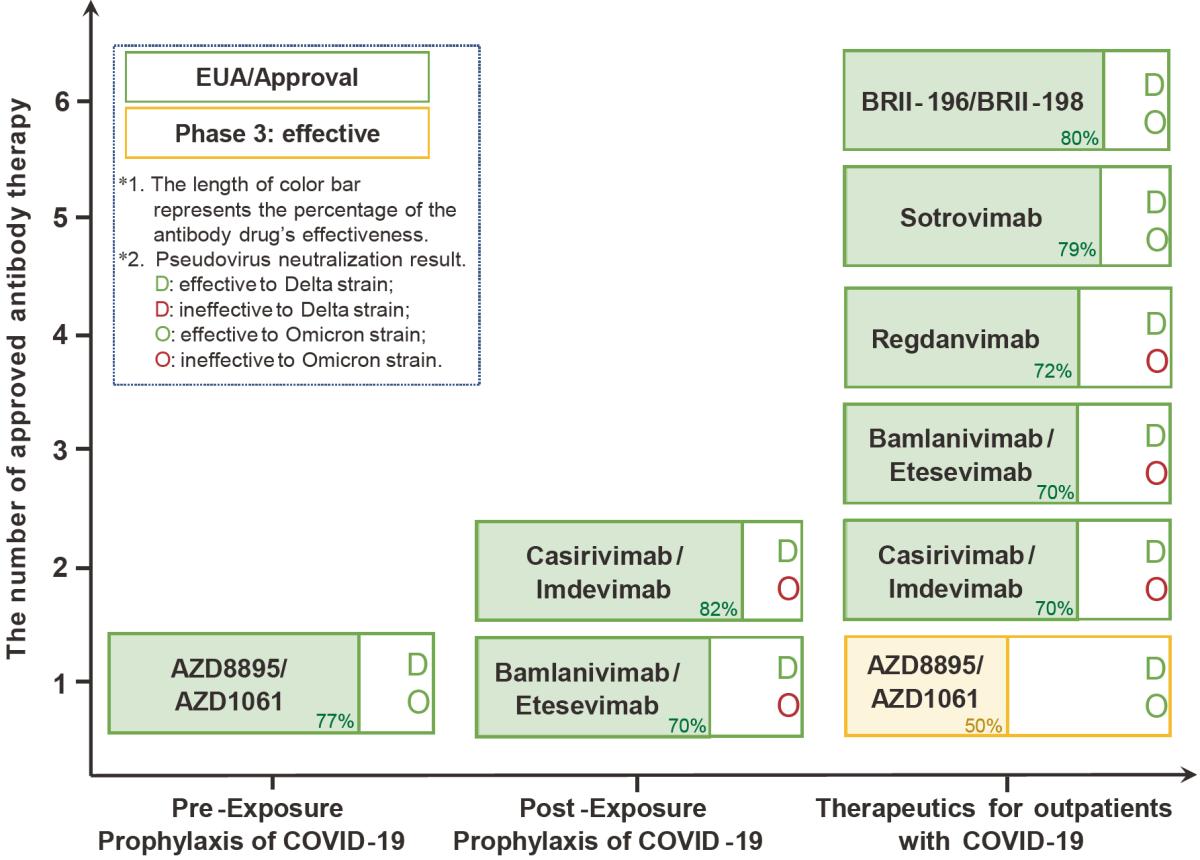
Summary of clinical efficacy for licensed antibodies or under EUA. Bamlanivimab/Etesevimab are combination of monoclonal antibody products developed by Lilly, Casirivimab/Imdevimab by Regeneron, Tixagevimab/Cilgavimab (AZD8895/AZD1061) by AstraZeneca, BRII-196/BRII-198 by Brii Biosciences, and CT-P59 (Regdanvimab) by Celltrion. The first three combinations have been approved by the U.S. Food and Drug Administration, the fourth one by the Chinese National Medical Products Administration, and the last one by South Korea regulatory agencies.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

