| Issue |
Natl Sci Open
Volume 4, Number 6, 2025
|
|
|---|---|---|
| Article Number | 20250064 | |
| Number of page(s) | 15 | |
| Section | Materials Science | |
| DOI | https://doi.org/10.1360/nso/20250064 | |
| Published online | 05 November 2025 | |
RESEARCH ARTICLE
Engineering the Si/Al ratio of MWW zeolites for propylene/propane separation
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemical Engineering, Nanjing Tech University, Nanjing 211816, China
* Corresponding authors (emails: This email address is being protected from spambots. You need JavaScript enabled to view it.
(Xiaoling Liu); This email address is being protected from spambots. You need JavaScript enabled to view it.
(Jun Wang); This email address is being protected from spambots. You need JavaScript enabled to view it.
(Yu Zhou))
Received:
8
October
2025
Revised:
31
October
2025
Accepted:
3
November
2025
Propylene/propane (C3H6/C3H8) separation is critical to the petrochemical industry but remains highly energy-consuming. Adsorption-based strategies provide a promising energy-efficient alternative, yet developing adsorbents combining both high capacity and selectivity is challenging due to the nearly identical physicochemical properties of C3H6 and C3H8. Here, we systematically investigated MCM-22 zeolites (MWW topology) with varying Si/Al ratios (9.6–36.5) for C3H6/C3H8 separation. Gas adsorption isotherms, cyclic sorption tests, and dynamic breakthrough experiments revealed that the Si/Al ratio significantly regulated the separation performance. Remarkably, MCM-22(30) with a moderate Si/Al ratio of 18.7 achieved the best performance, achieving a high C3H6 uptake (6.28 mmol g−1) at 298 K and 1 bar (1 bar = 105 Pa), with an exceptional ideal adsorption solution theory (IAST) C3H6/C3H8 (50/50, v/v) selectivity exceeding 3000, while maintaining favorable regenerability and structural stability over multiple cycles. Breakthrough experiments further demonstrated the superior dynamic separation efficiency of MCM-22(30) compared with its higher- or lower-Si/Al counterparts.
Key words: propylene purification / adsorption and separation / zeolites / hydrothermal synthesis / structural modulation
© The Author(s) 2025. Published by Science Press and EDP Sciences.
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Propylene (C3H6) is one important industrial chemical serving as a key feedstock for producing polypropylene, acrylonitrile, propylene oxide, and numerous other high-value chemicals [1−4]. The growing demand for polymer-grade propylene necessitates its efficient separation from propane (C3H8) [2,5]. At present, cryogenic distillation remains the dominant industrial technology; however, it is among the most energy-intensive processes in the chemical industry due to the extremely close boiling points of C3H6 and C3H8 (−47.6 °C vs. −42.1 °C) [1,4,6]. This substantial energy burden has driven increasing interest in the development of alternative, energy-saving separation technologies [7].
Among the emerging approaches, adsorption-based separation has gained growing attention owing to its low energy demand, mild operating conditions, and facile regenerability [8–10]. The major challenge, however, lies in designing adsorbents that simultaneously achieve high adsorption capacity and selectivity, given the nearly identical physicochemical properties and comparable molecular sizes of C3H6 (0.468 × 0.457 nm) and C3H8 (0.502 × 0.502 nm) [11–13]. Various porous materials have been investigated for C3H6/C3H8 separation, such as metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and carbon-based materials, and zeolite [13–17]. In particular, MOFs have demonstrated exceptional performance for C3H6/C3H8 separation because of their structural diversity and high tunability with respect to pore dimensions and functionality [2,5]. For example, Cu10O13-based MOF with a water nanotube within the channel exhibited a high C3H6/C3H8 selectivity of 1570 at 298 K and 1 bar [6]. Among these materials, zeolites are a family of low-cost inorganic crystals with excellent thermal and hydrothermal stability, well-defined pore architectures, and tunable chemical composition. Owing to these virtues, zeolites have been widely applied as adsorbents in industry, and in particular, LiX have been utilized in air separation to produce high-purity O2 and N2 via pressure swing adsorption (PSA) [18].
The ordered channel systems of zeolites enable molecular discrimination through subtle differences in size, shape, and framework interactions [11,19]. In general, small-pore zeolites (e.g., 8-membered-ring frameworks) exhibit high C3H6/C3H8 selectivity via molecular sieving or diffusion control, as their pore apertures are comparable to the kinetic diameters of C3H6 and C3H8 [20–23]. For example, pure-silica ITQ-3, chabazite (CHA), and high silica ZSM-58 (DDR) with 8-membered-ring channels have demonstrated distinct diffusion rates for C3H6 and C3H8, evidencing strong kinetic selectivity toward C3H6 [22,23]. However, the application of small pore zeolites is often hindered by their low adsorption capacities and severe diffusion limitations [24–26]. In contrast, large-pore zeolites provide higher uptake and faster mass transfer but generally suffer from poor selectivity because their pore apertures are much larger than the molecular dimensions of C3H6 and C3H8 [27–29]. Therefore, achieving an optimal balance between pore confinement, adsorption strength, and molecular accessibility remains a key challenge.
The adsorption and separation behavior of zeolites is strongly influenced by topology, Si/Al ratio, morphology, and overall composition [30–33]. High-silica zeolites are often preferred to mitigate pore blockage caused by olefin oligomerization, making Si/Al ratio engineering one of the most effective strategies for tailoring adsorption performance [21,22,31,34]. Among the diverse zeolite families, the MWW-type framework has recently emerged as a promising candidate for physical adsorbent [35,36]. With its two-dimensional sinusoidal 10-membered-ring channels and large super-cages accessible through 12-membered-ring opening, the layered MWW structure integrates the benefits of both small and large pore zeolites, generating a multi-functional adsorption environment [37,38]. Nevertheless, systematic studies on MWW zeolites for C3H6/C3H8 separation remain limited. In particular, the influence of the Si/Al ratio on adsorptive separation performance, cycling stability, and dynamic breakthrough behavior has yet to be thoroughly elucidated.
Here, we synthesized a series of MCM-22 zeolites with tunable Si/Al ratios (9.6–36.5) and systematically evaluated their C3H6/C3H8 separation performance. A combination of static gas adsorption, cyclic adsorption-desorption, and breakthrough experiments was employed to elucidate the influence of framework composition on separation efficiency. Systematic investigations revealed that the moderate Si/Al ratio delivers both high adsorption capacity and selectivity. The optimal adsorbent MCM-22(30) with a moderate Si/Al ratio of 18.7 exhibited the best performance, achieving a C3H6 uptake of 6.28 mmol g−1 and a C3H6/C3H8 selectivity above 3000 for an equimolar mixture at 298 K. Moreover, MCM-22(30) maintained structural stability and separation efficiency after multiple sorption cycles. Breakthrough tests further confirmed the superior dynamic selectivity of MCM-22(30) for C3H6 over C3H8.
RESULTS AND DISCUSSION
Synthesis and characterization
Three MWW-type zeolites with variable Si/Al ratios were synthesized hydrothermally and designated as MCM-22(n), where n = 15, 30, and 60 denotes the theoretical Si/Al ratio in the gel. The experimental detected Si/Al ratios were 9.6, 18.7, and 36.5 for n = 15, 30, and 60, respectively (Table 1). The chemical compositions of MCM-22(15), MCM-22(30), and MCM-22(60) were [Na0.68H6.12][Al6.8Si65.2O144], [Na0.29H3.41][Al3.7Si68.3O144], and [Na0.06H1.84][Al1.9Si70.1O144], respectively. The experimental measured values were consistently lower than the nominal gel ratios, which can be attributed to the different reactivity and solubility of silica and aluminum species during crystallization [39]. Particularly, the {L-End}
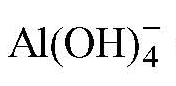 anion effectively compensates the framework negative charge and is more readily incorporated into the zeolite framework, resulting in a reduced Si/Al ratio. All the X-ray diffraction (XRD) patterns exhibited the characteristic reflections of the MWW topology (Figure 1a). MCM-22(n) crystallized in a hexagonal system with the space group P6/mmm. Prominent peaks in the low-angle region (2θ ≈ 6°–10°) correspond to layered structural reflections (e.g., (002), (100), and (hydrophobicity)), while multiple reflections in the mid-angle region (2θ ≈ 12°–13° and 22°–25°) are assigned to the ordered framework planes [40,41]. The similarity in diffraction peak positions across the three samples confirms that the MWW framework was successfully formed irrespective of Si/Al ratio. No impurity phases were detected, indicating high phase purity. Among the samples, MCM-22(30) displayed the sharpest and most intense reflections, implying the highest crystallinity and layer stacking order. Thermogravimetric (TG) analysis revealed gradual weight loss upon heating from room temperature to 800 °C (Figure 1b). All samples showed an initial weight loss below 200 °C, corresponding to the removal of physically adsorbed water (9.0%, 6.7%, and 3.6% for n = 15, 30, and 60, respectively). A subsequent minor loss up to ~600 °C (1.7%, 0.9%, and 0.45%) was mainly assigned to water desorption from the condensation of residual framework hydroxyl groups. The total weight loss decreased as the Si/Al ratio increased. Specially, MCM-22(15) with the lowest Si/Al ratio exhibited the largest overall loss (10.7% up to ~600 °C), followed by MCM-22(30) (7.6%), while MCM-22(60) presented the smallest (4.4%). This trend reflects the enhanced hydrophobicity at higher Si/Al ratio, which reduces the amount of adsorbed water and hydroxyl species.
anion effectively compensates the framework negative charge and is more readily incorporated into the zeolite framework, resulting in a reduced Si/Al ratio. All the X-ray diffraction (XRD) patterns exhibited the characteristic reflections of the MWW topology (Figure 1a). MCM-22(n) crystallized in a hexagonal system with the space group P6/mmm. Prominent peaks in the low-angle region (2θ ≈ 6°–10°) correspond to layered structural reflections (e.g., (002), (100), and (hydrophobicity)), while multiple reflections in the mid-angle region (2θ ≈ 12°–13° and 22°–25°) are assigned to the ordered framework planes [40,41]. The similarity in diffraction peak positions across the three samples confirms that the MWW framework was successfully formed irrespective of Si/Al ratio. No impurity phases were detected, indicating high phase purity. Among the samples, MCM-22(30) displayed the sharpest and most intense reflections, implying the highest crystallinity and layer stacking order. Thermogravimetric (TG) analysis revealed gradual weight loss upon heating from room temperature to 800 °C (Figure 1b). All samples showed an initial weight loss below 200 °C, corresponding to the removal of physically adsorbed water (9.0%, 6.7%, and 3.6% for n = 15, 30, and 60, respectively). A subsequent minor loss up to ~600 °C (1.7%, 0.9%, and 0.45%) was mainly assigned to water desorption from the condensation of residual framework hydroxyl groups. The total weight loss decreased as the Si/Al ratio increased. Specially, MCM-22(15) with the lowest Si/Al ratio exhibited the largest overall loss (10.7% up to ~600 °C), followed by MCM-22(30) (7.6%), while MCM-22(60) presented the smallest (4.4%). This trend reflects the enhanced hydrophobicity at higher Si/Al ratio, which reduces the amount of adsorbed water and hydroxyl species.
 |
Figure 1 (a) XRD patterns, (b) TG profiles, (c) N2 sorption isotherms, and (d) pore size distribution curves of MCM-22(n). |
Textural properties
The textural properties of MCM-22(n) were determined by nitrogen sorption experiment at 77 K. All samples displayed type I+IV isotherms (Figure 1c), indicative of predominant microporosity along with minor mesoporosity [42,43]. The steep uptake at low relative pressures corresponds to micropore filling, whereas the slight hysteresis loop at higher relative pressures reflects secondary mesopores formed by interparticle packing, which is further reflected by the pore size distribution curves (Figure 1d). The corresponding surface areas and pore volumes are listed in Table 1. All of them showed high surface area and pore volume, with the values of 546 m2 g−1 and 0.23 cm3 g−1 for MCM-22(15), 594 m2 g−1 and 0.26 cm3 g−1 for MCM-22(30), and 550 m2 g−1 and 0.23 cm3 g−1 for MCM-22(60). The overall similarity of the isotherms indicates that the intrinsic microporosity of the MWW framework was preserved, while minor variations in surface area like arise from subtle differences in crystal size and stacking.
The surface wettability of MCM-22(n) was assessed by a water contact angle test (Figure 2a–c). The water contact angles of MCM-22(15), MCM-22(30), and MCM-22(60) were 19.1°, 28.8°, and 53.9°, respectively, indicating that increasing the Si/Al ratio reduces hydrophilicity due to a lower density of surface hydroxyl groups. Scanning electron microscope (SEM) images of MCM-22(n) exhibited typical aggregated platelet-like morphologies characteristic of MWW structure (Figure 2d–g). The primary particles were nanosheets with the size from 100 to 300 nm (Figure 2). They were randomly packed with each other to give aggregations at the micrometer level. MCM-22(15) showed relatively compact aggregates with tightly stacked layers, whereas MCM-22(30) and MCM-22(60) exhibited more open assemblies with discernible intercrystalline voids and aggregation density without altering the primary platelet morphology [39,41]. The Fourier transform infrared (FT-IR) spectra of MCM-22(n) displayed the typical vibrational features of the MWW framework (Figure S1). Broad bands at 3430–3440 cm−1 are assigned to O–H stretching vibrations of surface hydroxyl groups and adsorbed water, with bending modes near 1636–1660 cm−1 [44–46]. Strong absorption at ~1090–1050 cm−1, together with bands at 791–768 and 447–444 cm−1, corresponds to the asymmetric and symmetric Si–O–Si stretching and bending vibrations [47]. The characteristic band at ~617–610 cm−1 comes from the double six-membered-ring (D6R) units, a fingerprint of the MWW structure [44,48]. All three samples showed similar spectra, confirming structural uniformity [40], while the intensity of the hydroxyl-related bands decreased with increasing Si/Al ratio, consistent with the enhanced hydrophobicity of high-silica samples.
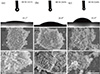 |
Figure 2 Water contact angles of (a) MCM-22(15), (b) MCM-22(30), and (c) MCM-22(60). SEM images of (d, g) MCM-22(15), (e, h) MCM-22(30), and (f, i) MCM-22(60). |
Gas adsorption
Single-component adsorption isotherms of C3H6 and C3H8 on MCM-22(n) samples were collected using a volumetric adsorption analyzer (Figure 3). The C3H6 adsorption isotherms at 298 K (Figure 3a) exhibited typical type I+IV behavior, characterized by a steep at low relative pressures [49]. MCM-22(15) showed a gradual increase in uptake with increasing pressure, reaching a C3H6 uptake of 4.45 mmol g−1 at 1 bar. As the Si/Al ratio increased to 30, the adsorption capacity significantly improved over the entire pressure range, attributable to the higher crystallinity and larger surface area of MCM-22(30). A maximum C3H6 uptake of 6.28 mmol g−1 was reached at 1 bar (1 bar = 105 Pa). However, at a higher Si/Al ratio of 60, the sample exhibited a reduced uptake (3.95 mmol g−1). Considering that MCM-22(60) has almost same textural properties to MCM-22(15) (546 vs. 550 m2 g−1 and 0.23 vs. 0.23 cm3 g−1), the decline can be assigned to the decreased framework Al density and weakened electrostatic field strengthen, which diminish the host-guest interactions between C3H6 molecules and the zeolites channels [31,50]. The C3H8 adsorption isotherms at 298 K (Figure 3b) display similar shapes for all three samples, with uptake capacities of 2.07, 2.10, and 2.10 mmol g−1 at 1 bar for n = 15, 30, and 60, respectively (Table S1). The initial uptakes at low relative pressures are evident, but the overall adsorption of C3H8 is much weaker than that of C3H6, suggesting a weaker interaction between C3H8 molecules and the MCM-22 framework.
 |
Figure 3 (a) C3H6 and (b) C3H8 adsorption isotherms of MCM-22(n) at 298 K up to 1 bar. (c) C3H6 and (d) C3H8 adsorption isotherms of MCM-22(n) at 273 K up to 1 bar. IAST predictions of C3H6/C3H8 (50/50, v/v) selectivities of MCM-22(n) at (e) 298 K and (f) 273 K. |
These C3H6 and C3H8 adsorption isotherms were fitted by using different equations. High agreement was achieved by using the Dual-Langmuir (DL) equation [30], indicating the presence of two distinct adsorption sites for both C3H6 and C3H8 on MCM-22(n) (Figures S2–S13). The fitted parameters revealed that both the saturated capacities and equilibrium constants of C3H6 were larger than those of C3H8, confirming the stronger affinity of MCM-22 for C3H6 adsorption. Based on these fitted parameters, the ideal adsorption solution theory (IAST) was applied to predict the C3H6/C3H8 selectivity for the separation of an equimolar (50/50, v/v) mixture (Figure 3e) [51]. The calculated C3H6/C3H8 selectivities increased with pressure for all samples. Among them, MCM-22(15) exhibited a selectivity of 1846 at 1 bar, which further increased to 3553 for MCM-22(30), demonstrating that increasing the Si/Al ratio enhances both C3H6 uptake and C3H6/C3H8 selectivity (Table S1). Further increasing the Si/Al ratio to 60, however, reduced the C3H6/C3H8 selectivities due to the weakened C3H6-zeolite interaction [50]. The selective adsorption performance of MCM-22(30) is superior or at least comparable to reported C3H6-selective porous materials with similar pore sizes [6,20,52,53].
The temperature-dependent adsorption behavior was further investigated at 273 K (Figure 3c and d). The isotherm profiles of both C3H6 and C3H8 remained similar to those at 298 K. For C3H6 adsorption (Figure 3c), only minor variations were observed between 273 and 298 K, with uptakes of 4.49 vs. 4.45 mmol g−1 for MCM-22(15), 6.33 vs. 6.28 mmol g−1 for MCM-22(30), and 4.27 vs. 3.95 mmol g−1 for MCM-22(60). This suggests that C3H6 adsorption is only weakly temperature-dependent. In contrast, C3H8 adsorption increased noticeably at the lower temperature (Figure 3d), with uptakes of 2.63 vs. 2.07 mmol g−1 for MCM-22(15), 2.50 vs. 2.10 mmol g−1 for MCM-22(30), and 2.55 vs. 2.10 mmol g−1 for MCM-22(60), reflecting a stronger thermodynamic driving force for C3H8 adsorption at 273 K. Consequently, the calculated C3H6/C3H8 (50/50, v/v) selectivities decreased at 273 K, with MCM-22(30) maintaining the highest value of 181, followed by MCM-22(15) (57) and MCM-22(60) (13) (Figure 3f). Considering that industrial separations are generally performed near ambient temperature, the weak temperature dependence of C3H6 adsorption on MCM-22(30) highlights its suitability for practical applications.
Five-cycle adsorption--desorption recyclability tests confirmed good recyclability (Figure 4). After each run, the samples were reactivated under vacuum activated before reuse. For C3H6 adsorption, the isotherm shapes remained nearly identical throughout the cycles (Figure 4a–c). A moderate decline in uptake occurred between the first and second runs, particularly for MCM-22(15), where the uptake decreased from 4.45 to 2.57 mmol g−1. Thereafter, the capacities stabilized with negligible loss ever after the 5th cycle. MCM-22(30) retained a high C3H6 uptake (> 5 mmol g−1 in the 5th run), outperforming both MCM-22(15) (2.53 mmol g−1) and MCM-22(60) (3.37 mmol g−1) (Table S1). In contrast, the C3H8 adsorption isotherms showed almost overlapping profiles across all cycles (Figure 4b), confirming good reversibility and stability. The C3H8 capacities in the 5th run were nearly identical to those in the 1st run for all samples, suggesting a negligible influence of Si/Al ratio on cycling C3H8 adsorption. The evolution of C3H6/C3H8 selectivity during cycling further highlights the importance of the Si/Al ratio. For MCM-22(15), selectivity drastically decreased from 1846 in the 1st run to 3.6 in the 2nd run, and stabilized around 2 thereafter (Figure S14 and Table S1). At a high Si/Al ratio (MCM-22(60)), selectivity declined gradually over successive cycles, reaching only 2.7 after the 5th run (Figure S15 and Table S1). In contrast, MCM-22(30) maintained superior performance, with selectivity decreasing modestly from 3553 to 478 in the 2nd run and remaining above 300 in subsequent cycles (Figure S16 and Table S1). The sustained high C3H6 uptake and C3H6/C3H8 selectivity for MCM-22(30) confirms its favorable recycling adsorption-desorption operation.
 |
Figure 4 Recycling adsorption isotherms of C3H6 on (a) MCM-22(15), (b) MCM-22(30), (c) MCM-22(60), and C3H8 on (d) MCM-22(15), (e) MCM-22(30), (f) MCM-22(60). |
Structure of spent MWW zeolites
Structural characterizations were conducted on the spent MCM-22(n) recovered after the 5th run of C3H6 adsorption, denoted as MCM-22(n)-5th. Their XRD patterns retained the characteristic reflections of the MWW topology (Figure 5a), corroborating that the crystalline structure was preserved after repeated sorption cycles. The sharp and well-defined diffraction peaks were observed in the low- and moderate-angle regions, with negligible position shifts or the emergence of impurity phases. The slight attenuation of diffraction intensities for the spent samples can be attributed to residual organic species, mainly the propylene oligomers, remaining within the channels, which reduce electron-density contrast between the framework walls and pores [2,54].
 |
Figure 5 (a) XRD patterns, (b) TG profiles, (c) N2 sorption isotherms, and (d) pore size distribution curves of spent MCM-22(n)-5th. |
TG profiles of MCM-22(n)-5th showed two distinct weight-loss regions (Figure 5b). The first below 200 °C corresponds to desorption of physiosorbed water, while the second (200–600 °C) arises from decomposition of residue organics. The initial weight loss followed the sequence 5.1% (n = 15) > 4.5% (n = 30) > 3.4% (n = 60), consistent with the improved hydrophobicity at higher Si/Al ratios. Compared with the fresh samples, all spent zeolites showed reduced initial weight loss, further indicating that residual organics increased surface hydrophobicity. The second weight loss (1.8%, 1.6%, and 1.4%) was larger than that of the pristine materials, validating the presence of adsorbed hydrocarbon residues from C3H6 sorption process. Elemental analysis of MCM-22(n)-5th was performed to quantify the hydrocarbon residue in Table S2. The carbon residue content of MCM-22(15) was higher than that of MCM-22(30) and MCM-22(60), consistent with the results of TG.
N2 sorption isotherms (Figure 5c) of MCM-22(n)-5th maintained the type IV features of the fresh samples, with significant micropore uptake at low p/p0 and a gradual rise at higher pressure. However, the uptakes at low relative pressures were notably reduced, while those at high pressure (p/p0 > 0.8) increased, suggesting the formation of secondary mesopores [44]. MCM-22(15)-5th exhibited the most pronounced decline in surface area and pore volume (546 to 234 m2 g−1 and 0.23 to 0.11 cm3 g−1) (Figure 5c, d, and Table S3), indicating partial pore blockage by retained organics. Increasing the Si/Al ratio mitigated these losses. MCM-22(30)-5th maintained 358 m2 g−1 surface area and 0.17 cm3 g−1 pore volume (Figure 5c, d, and Table S3), while MCM-22(60)-5th retained comparable values. These results reveal that moderate Si/Al ratios effectively preserve the pore structure and reduce hydrocarbon residue accumulation, accounting for the superior recyclability and sustained C3H6/C3H8 separation efficiency of MCM-22(30).
Dynamic separation and comparison
The dynamic C3H6/C3H8 separation performance of MCM-22 (n) was evaluated in the column breakthrough experiment using a gas mixture with 50% C3H6 and 50% C3H8 (Figure 6a and Table S4). All samples showed clear separation between the two components. For MCM-22(15), C3H8 broke through first at ~22 min g−1, followed by a sharp increase in its effluent concentration (C/C0 > 2.0) due to competitive adsorption, whereas C3H6 eluted much later (~44 min g−1). A similar trend was observed for MCM-22(60), where C3H8 and C3H6 broke through at 26 and 51 min g−1, respectively (Figure 6a). Remarkably, MCM-22(30) exhibited the best separation behavior, with breakthrough time of 65 min g−1 (for C3H8) and 151 min g−1 (for C3H6). The pronounced time delay between the two breakthrough fronts highlights the strong preferential adsorption of C3H6 and demonstrates the excellent selectivity of MCM-22(30) under dynamic flow conditions.
 |
Figure 6 (a) Breakthrough curves for the separation of a C3H6/C3H8 (50/50, v/v) mixture on MCM-22(n). (b) Comparison of IAST C3H6/C3H8 (50/50, v/v) selectivity and C3H6 uptake (mmol g−1) of MCM-22(n) with advanced C3H6-selective materials at 298 K and 1 bar. |
A comparative analysis (Figure 6b and Table S5) further benchmarked MCM-22(30) against representative porous adsorbents, including other zeolites, carbon materials, and MOFs. MCM-22 (30) surpasses most previously reported adsorbents in both C3H6 adsorption capacity and C3H6/C3H8 selectivity, confirming its superior separation efficiency and potential as a robust, energy-efficient material for olefin/paraffin separations [6,20,52,53].
CONCLUSIONS
MCM-22 zeolites with tailorable Si/Al ratios were synthesized, and their performance in C3H6/C3H8separation was systematically investigated. Structural characterization validated the formation of the typical MWW topology with high crystallinity. Increasing the Si/Al ratio decreases the framework acidity and surface hydroxyl density while enhancing hydrophobicity. Gas adsorption measurements demonstrated that MCM-22(30) with a moderate Si/Al ratio of 18.7 exhibited the highest C3H6 uptake of 6.28 mmol g−1 and C3H6/C3H8 (50/50, v/v) selectivity of 3553 at 298 K. Recycling adsorption-desorption tests revealed the favorable reversibility and structural integrity after multiple cycles. Column breakthrough experiments further evidenced the superior dynamic C3H6/C3H8 separation over MCM-22(30). This work highlights the great potential of MWW topologic zeolites in the C3H6/C3H8 separation, with a moderate Si/Al ratio offering the best performance.
METHOD
Materials
Silica sol was provided by Guangzhou Yinhuan Chemical (China). Sodium hydroxide (NaOH, 96.0 wt%) was offered by Xilong Chemical Reagent Co. (China). Sodium meta-aluminate (NaAlO2, 80 wt%) was purchased by Sinopharm Chemical Reagent Co. (China). Hexamethyleneimine (HMI, 99%) was obtained from Sinopharm Chemical Reagent Co. (China).
Materials synthesis
MWW-type zeolites were synthesized hydrothermally following a modified literature procedure [55]. Typically, NaOH (0.094 g), NaAlO2 (0.182), silica sol (4.2 g), and HMI (0.9 g) were subsequently dissolved in water (7.738 g), followed by stirring at room temperature for 3 h. The resulting gel with the molar composition of 1 SiO2:0.11 Na2O:0.03 Al2O3:0.43 HMI:20.48 H2O was dynamically crystallized at 150 °C under rotation (50 r min−1) for 5 d. The solid was recovered by filtration, washed thoroughly with water, and dried to yield as-synthesized MCM-22(15). Calcination was conducted at 550 °C for 5 h. MCM-22(30) and MCM-22(60) were synthesized using the same procedure with the NaAlO2 feeding of 0.091 and 0.046 g, respectively, to achieve higher Si/Al ratios.
General characterizations
XRD patterns were collected on a (Rigaku) SmartLab diffractometer with a 9 kW rotating Cu Kα anode (40 kV, 100 mA, 5°–50°, 0.2° s−1). FT-IR spectra were measured on an Agilent Cary 660 spectrometer (USA). Morphology was observed with a Hitachi S-4800 (Japan) field emission scanning electron microscope (SEM). N2 sorption experiment was conducted on a BELSORP-MAX analyzer (Japan) to determine textural parameters. Elemental composition was analyzed by an ADVANT’XP X-ray fluorescence (XRF) spectrometer (ZSX Primus II, Rigaku, Japan). TG analysis was performed on a STA409 equipment (NETZSCH, Germany) under N2. The water contact angles on the surface of samples were gauged with a contact angle goniometer (Powereach JC2000, China) equipped with uEye digital camera.
Gas adsorption
Single-component C3H6 and C3H8 sorption isotherms were measured at 298 and 273 K on a BELSORP-MAX apparatus (Japan). The equilibrium data were fitted using Dual-site Langmuir model, and the C3H6/C3H8 selectivities are calculated from IAST [49].
Breakthrough experiments
Breakthrough curves were collected on a custom-built stainless-steel fixed-bed apparatus. The zeolite-packed column (inner diameter: 5 mm; packing length: ~5 cm; zeolite loading: 0.3875 g for MCM-22(15), 0.4107 g for MCM-22(30), and 0.2529 g for MCM-22(60)) was pretreated at 300 °C under vacuum, followed by purging with He (5 mL min−1). After that, gas mixture of C3H6/C3H8 (50/50, v/v) was introduced at a flow rate of 2 mL min−1. The effluent composition was continuously monitored by online gas chromatography.
Acknowledgments
The computational resources generously provided by the High-Performance Computing Center of Nanjing Tech University are greatly appreciated.
Data availability
The original data are available from the corresponding authors upon reasonable request.
Funding
This work was supported by the National Natural Science Foundation of China (22222806, 22178162, 22408170, and 22072065), the Distinguished Youth Foundation of Jiangsu Province (BK20220053), the National Key Research and Development Program of China (2024YFE0206900), the Six Talent Peaks Project in Jiangsu Province (JNHB-035), and the State Key Laboratory of Materials-Oriented Chemical Engineering (SKL-MCE-24A06).
Author contributions
Z.T. and Q.X. contributed to writing the original draft, investigation, and data curation; Y.W., X.S., and X.Z. contributed to data curation; X.L., J.W., and Y.Z. contributed to writing review and editing, supervision, project administration, and funding acquisition.
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
Supplementary file provided by the authors. Access here
References
- Cui J, Zhang Z, Yang L, et al. A molecular sieve with ultrafast adsorption kinetics for propylene separation. Science 2024; 383: 179-183. [Article] [Google Scholar]
- Zhang L, Gao R, Liu X, et al. Unique H2O vortex in one new MOF material strengthening C3H6 adsorption and unprecedented purification from C2H4/C3H8/C3H6 mixtures. Adv Funct Mater 2025; 35: 2420927. [Article] [Google Scholar]
- Ma LL, Liu J, Zhou K, et al. A MOF built on 8-connected Y3 nodes demonstrates simultaneous reverse adsorptive separation: Ethane over ethylene and propane over propylene. Sci China Chem 2024; 67: 3657-3661. [Article] [Google Scholar]
- Chen XY, Xiao A, Rodrigue D. Polymer-based membranes for propylene/propane separation. Sep Purif Rev 2022; 51: 130-142. [Article] [Google Scholar]
- Lan T, Yu B, Liu Y, et al. Two-dimensional anion-pillared metal-organic framework for sieving separation of propylene from propane with ultrahigh kinetic performance. Inorg Chem 2025; 64: 5322-5330. [Article] [Google Scholar]
- Dong Q, Huang Y, Wan J, et al. Confining water nanotubes in a Cu10O13-based metal-organic framework for propylene/propane separation with record-high selectivity. J Am Chem Soc 2023; 145: 8043-8051. [Article] [Google Scholar]
- Ding Q, Zhang S. Recent advances in the development of metal-organic frameworks for propylene and propane separation. Energy Fuels 2022; 36: 7337-7361. [Article] [Google Scholar]
- Moliner M, Martínez C, Corma A. Synthesis strategies for preparing useful small pore zeolites and zeotypes for gas separations and catalysis. Chem Mater 2014; 26: 246-258. [Article] [Google Scholar]
- Abbasi S, Khosravi-Nikou MR, Shariati A. Selective separation of propane from the propylene-propane mixture using pure silica zeolites: A molecular dynamic simulation. Chem Eng Process Int 2023; 184: 109294. [Article] [Google Scholar]
- Jia J, Yan N, Lian X, et al. Molecular trapdoor in HEU zeolite enables inverse CO2-C2H2 separation. Angew Chem Int Ed 2025; 64: e202419091. [Article] [Google Scholar]
- Bai R, Song X, Yan W, et al. Low-energy adsorptive separation by zeolites. Natl Sci Rev 2022; 9: nwac064. [Article] [Google Scholar]
- Yue B, Liu S, Chai Y, et al. Zeolites for separation: Fundamental and application. J Energy Chem 2022; 71: 288-303. [Article] [Google Scholar]
- Wang Y, Peh SB, Zhao D. Alternatives to cryogenic distillation: Advanced porous materials in adsorptive light olefin/paraffin separations. Small 2019; 15: 1900058. [Article] [Google Scholar]
- Wei R, Liu X, Lai Z. MOF or COF membranes for olefin/paraffin separation: Current status and future research directions. Adv Membr 2022; 2: 100035. [Article] [Google Scholar]
- Xu S, Liu RS, Zhang MY, et al. Designed synthesis of porous carbons for the separation of light hydrocarbons. Chin J Chem Eng 2022; 42: 130-150. [Article] [Google Scholar]
- Zhou Y, Li P, Wang Y, et al. Progress in the separation and purification of carbon hydrocarbon compounds using MOFs and molecular sieves. Separations 2023; 10: 543. [Article] [Google Scholar]
- Tian YJ, Deng C, Peng YL, et al. State of the art, challenges and prospects in metal-organic frameworks for the separation of binary propylene/propane mixtures. Coord Chem Rev 2024; 506: 215697. [Article] [Google Scholar]
- Jeong SR, Park H, Ko Y, et al. Adsorption characteristics and separation behavior of binder and binderless zeolite LiX Pellets: O2, N2, and CO2. Sep Purif Tech 2025; 355: 129702. [Article] [Google Scholar]
- Boer DG, Langerak J, Pescarmona PP. Zeolites as selective adsorbents for CO2 separation. ACS Appl Energy Mater 2023; 6: 2634-2656. [Article] [Google Scholar]
- Maghsoudi H, Abdi H, Aidani A. Temperature- and pressure-dependent adsorption equilibria and diffusivities of propylene and propane in pure-silica Si-CHA zeolite. Ind Eng Chem Res 2020; 59: 1682-1692. [Article] [Google Scholar]
- Moradi H, Azizpour H, Mohammadi M. Study of adsorption of propane and propylene on CHA zeolite in different Si/Al ratios using molecular dynamics simulation. Powder Tech 2023; 419: 118329. [Article] [Google Scholar]
- Olson DH, Camblor MA, Villaescusa LA, et al. Light hydrocarbon sorption properties of pure silica Si-CHA and ITQ-3 and high silica ZSM-58. Micropor Mesopor Mat 2004; 67: 27-33. [Article] [Google Scholar]
- Khalighi M, Chen YF, Farooq S, et al. Propylene/propane separation using SiCHA. Ind Eng Chem Res 2013; 52: 3877-3892. [Article] [Google Scholar]
- Grande CA, Rodrigues AE. Propane/propylene separation by pressure swing adsorption using zeolite 4A. Ind Eng Chem Res 2005; 44: 8815-8829. [Article] [Google Scholar]
- Chen Y, Jiang H, Peng Q, et al. Successively separating C3H6 and C2H4 from C2H4/C2H6/C3H6/C3H8 mixture in tandem fixed beds involving two zeolites LTA well-regulated via low transition-metal doping. Chem Eng J 2023; 473: 145151. [Article] [Google Scholar]
- Kencana KS, Hong SB. Thermodynamic-kinetic synergistic separation of light olefins/paraffins in post-synthetically modified small-pore zeolites. Small 2025; 21: e06212. [Article] [Google Scholar]
- Xiong Y, Tian T, L’Hermitte A, et al. Using silver exchange to achieve high uptake and selectivity for propylene/propane separation in zeolite Y. Chem Eng J 2022; 446: 137104. [Article] [Google Scholar]
- Granato MA, Jorge M, Vlugt TJH, et al. Diffusion of propane, propylene and isobutane in 13X zeolite by molecular dynamics. Chem Eng Sci 2010; 65: 2656-2663. [Article] [Google Scholar]
- Lee HS, Kim NS, Kwon D, et al. Post-synthesis functionalization enables fine-tuning the molecular-sieving properties of zeolites for light olefin/paraffin separations. Adv Mater 2021; 33: 2105398. [Article] [Google Scholar]
- Deng G, Cheng K, Meng B, et al. Regulating the Si/Al ratio of GIS zeolite with bulky primary particles for selective CO2 capture from hydrocarbons. Sep Purif Tech 2024; 340: 126764. [Article] [Google Scholar]
- Li Y, Wang Y, Bai H, et al. Adjusting the Si/Al ratio of high silica zeolites for efficient ethane and ethylene separation. Sep Purif Tech 2024; 336: 126154. [Article] [Google Scholar]
- Vosoughi M, Maghsoudi H. Characterization of size-selective kinetic-based Ba-ETS-4 titanosilicate for nitrogen/methane separation: Chlorine-enhanced steric effects. Sep Purif Tech 2022; 284: 120243. [Article] [Google Scholar]
- Park J, Cho KH, Kim JC, et al. Design of olefin-phobic zeolites for efficient ethane and ethylene separation. Chem Mater 2023; 35: 2078-2087. [Article] [Google Scholar]
- Zhu W, Kapteijn F, Moulijn JA, et al. Shape selectivity in adsorption on the all-silica DD3R. Langmuir 2000; 16: 3322-3329. [Article] [Google Scholar]
- Fu H, Qin H, Wang Y, et al. Adsorption and separation of n/iso-pentane on zeolites: A GCMC study. J Mol Graphics Model 2018; 80: 59-66. [Article] [Google Scholar]
- Akyalçın S, Akyalçın L. Investigation of hydrogen uptake capacity for FAU, MOR, MTW, MWW. Int J Hydrogen Energy 2024; 80: 771-778. [Article] [Google Scholar]
- Zou Z, Kou C, E J, et al. Experimental and simulation investigation on the hydrocarbon adsorption performance of the transition metal ion-modified zeolite during cold start process of automotive engine. Energy 2025; 330: 137012. [Article] [Google Scholar]
- Du H, Kalyanaraman M, Camblor MA, et al. Hydrocarbon sorption properties of pure silica MCM-22 type zeolite. Micropor Mesopor Mat 2000; 40: 305-312. [Article] [Google Scholar]
- Delitala C, Alba MD, Becerro AI, et al. Synthesis of MCM-22 zeolites of different Si/Al ratio and their structural, morphological and textural characterisation. Micropor Mesopor Mat 2009; 118: 1-10. [Article] [Google Scholar]
- Diao D, Zhang H, Wang J, et al. Wet-oxidization and exfoliation of non-swollen MCM-22P to dispersible two-dimensional MWW zeolite nanosheets. Micropor Mesopor Mat 2022; 330: 111629. [Article] [Google Scholar]
- Chen JQ, Li YZ, Hao QQ, et al. Controlled direct synthesis of single- to multiple-layer MWW zeolite. Natl Sci Rev 2021; 8: nwaa236. [Article] [Google Scholar]
- Sachse A, Aumond T, Rousseau J, et al. Impact of hierarchization on the textural properties of MCM-22 based zeolites. Adv Mater Inter 2021; 8: 2100356. [Article] [Google Scholar]
- Li H, Zhang C, Lin Q, et al. Epitaxial growth of two-dimensional MWW zeolite. J Am Chem Soc 2024; 146: 8520-8527. [Article] [Google Scholar]
- Shamzhy M, Gil B, Opanasenko M, et al. MWW and MFI frameworks as model layered zeolites: Structures, transformations, properties, and activity. ACS Catal 2021; 11: 2366-2396. [Article] [Google Scholar]
- Cao SW, Xiao P, Wang J, et al. Active precursor promoting nucleation/growth of MWW zeolite and controlling its morphology. Pet Sci 2023; 20: 1922-1933. [Article] [Google Scholar]
- He WJ, Shen JX, Zhang KB, et al. Incorporation of Cu(I) sites into zeolite via a controllable reduction strategy for ethylene/ethane separation. Inorg Chem 2025; 64: 6236-6242. [Article] [Google Scholar]
- Liu S, Wu G, Gong J, et al. Synthesis gold and jade type core shell structure Pt@Sn in deboronated MWW zeolite and its good performance for light alkane dehydrogenation. Chem Eng J 2023; 476: 146410. [Article] [Google Scholar]
- Li Q, Cong W, Li K, et al. Transformation synthesis of SSZ-13 zeolite from ZSM-35 zeolite. J Solid State Chem 2021; 304: 122635. [Article] [Google Scholar]
- Zhou Y, Hu W, Xi S, et al. Mn-doped Y zeolite for ethylene/acetylene separation. Chem Eng Sci 2025; 316: 121960. [Article] [Google Scholar]
- Min JG, Kemp KC, Hong SB. Propylene/propane separation on a ferroaluminosilicate levyne zeolite. Micropor Mesopor Mat 2020; 294: 109833. [Article] [Google Scholar]
- Zhou Y, Zhang J, Wang L, et al. Self-assembled iron-containing mordenite monolith for carbon dioxide sieving. Science 2021; 373: 315-320. [Article] [Google Scholar]
- Wang CT, Li WC, Wang M, et al. Wood-structured carbon with reassembled pores showing high propylene adsorption rate for efficient separation of propylene/propane. Sep Purif Tech 2025; 354: 128649. [Article] [Google Scholar]
- Gi Min J, Christian Kemp K, Kencana KS, et al. Dealuminated Cs-ZK-5 zeolite for propylene/propane separation. Chem Eng J 2021; 413: 127422. [Article] [Google Scholar]
- Koudelková E, Ghrib Y, de Oliveira Ramos FS, et al. Adsorption and separation of the C3 hydrocarbons on cationic FER zeolites: Effect of dual sites existence. Micropor Mesopor Mat 2019; 279: 416-422. [Article] [Google Scholar]
- Tuo J, Sheng Z, Gong X, et al. Shape-selective synthesis of para-xylene through tandem CO2 hydrogenation and toluene methylation over ZnCeZrO/MCM-22 catalyst. Chin J Catal 2025; 73: 174-185. [Article] [Google Scholar]
All Tables
All Figures
 |
Figure 1 (a) XRD patterns, (b) TG profiles, (c) N2 sorption isotherms, and (d) pore size distribution curves of MCM-22(n). |
| In the text | |
 |
Figure 2 Water contact angles of (a) MCM-22(15), (b) MCM-22(30), and (c) MCM-22(60). SEM images of (d, g) MCM-22(15), (e, h) MCM-22(30), and (f, i) MCM-22(60). |
| In the text | |
 |
Figure 3 (a) C3H6 and (b) C3H8 adsorption isotherms of MCM-22(n) at 298 K up to 1 bar. (c) C3H6 and (d) C3H8 adsorption isotherms of MCM-22(n) at 273 K up to 1 bar. IAST predictions of C3H6/C3H8 (50/50, v/v) selectivities of MCM-22(n) at (e) 298 K and (f) 273 K. |
| In the text | |
 |
Figure 4 Recycling adsorption isotherms of C3H6 on (a) MCM-22(15), (b) MCM-22(30), (c) MCM-22(60), and C3H8 on (d) MCM-22(15), (e) MCM-22(30), (f) MCM-22(60). |
| In the text | |
 |
Figure 5 (a) XRD patterns, (b) TG profiles, (c) N2 sorption isotherms, and (d) pore size distribution curves of spent MCM-22(n)-5th. |
| In the text | |
 |
Figure 6 (a) Breakthrough curves for the separation of a C3H6/C3H8 (50/50, v/v) mixture on MCM-22(n). (b) Comparison of IAST C3H6/C3H8 (50/50, v/v) selectivity and C3H6 uptake (mmol g−1) of MCM-22(n) with advanced C3H6-selective materials at 298 K and 1 bar. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

