| Issue |
Natl Sci Open
Volume 4, Number 1, 2025
Special Topic: Nuclear Environment Advances
|
|
|---|---|---|
| Article Number | 20240020 | |
| Number of page(s) | 17 | |
| Section | Earth and Environmental Sciences | |
| DOI | https://doi.org/10.1360/nso/20240020 | |
| Published online | 31 October 2024 | |
RESEARCH ARTICLE
Huge block adsorbent with super-sized water transport channels for ultrafast and high-capacity recovery of dispersed uranium
State Key Laboratory of Marine Resource Utilization in South China Sea, Collaborative Innovation Center of Marine Science and Technology, Hainan University, Haikou 570228, China
* Corresponding authors (emails: This email address is being protected from spambots. You need JavaScript enabled to view it.
(Yihui Yuan); This email address is being protected from spambots. You need JavaScript enabled to view it.
(Ning Wang))
Received:
23
May
2024
Revised:
5
September
2024
Accepted:
19
September
2024
Abstract
Efficient recovery of dispersed uranium is critical for the sustainable development of nuclear energy. Designing an adsorbent with water transport channel structure is a promising approach for boosting uranium recovery performance, while balancing the demands of high stability and high adsorption capacity using currently available structural design methods is challenging. Herein, a novel structurally stable huge block sponge with super-sized water transport channels (SWTC-PAO sponge) is fabricated to recover dispersed uranium. In natural seawater, SWTC-PAO sponge realizes a high uranium extraction capacity of 9.1 mg g−1 with a rapid extraction rate. Moreover, the SWTC-PAO sponge can efficiently remove 99.59% of uranium within 24 h from uranium-containing wastewater. The concentrations of metal ions in the treated wastewater are lower than the drinking water standard set by the WHO. The broad application potential in natural seawater and wastewater makes the SWTC-PAO sponge a highly promising adsorbent for recovering dispersed uranium resources and for treating environmental uranium pollution.
Key words: uranium / adsorbent / water transport channels / seawater / wastewater
© The Author(s) 2024. Published by Science Press and EDP Sciences.
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Nuclear energy is considered a vital source of clean and efficient energy, significantly contributing to the global transition from traditional fossil fuels to sustainable energy sources [1–4]. Sustainable access to uranium, the critical raw material for fueling nuclear plants, is pivotal in supporting the sustainable development of nuclear energy [5,6]. Nevertheless, the foreseeable depletion of terrestrial uranium resources within the next century, coupled with their concentration in a limited number of countries, has raised concerns regarding potential supply shortages [7,8]. The ocean contains an estimated 4.5 billion tons of uranium, significantly exceeding the known reserves of terrestrial uranium ores, thereby presenting potential as an almost inexhaustible supply of uranium [9–11]. Consequently, innovative strategies for uranium acquisition are urgently required. In addition, several processes in nuclear energy generation, including uranium ore mining, uranium ore smelting, and spent fuel processing, have led to the generation of significant amounts of uranium-containing wastewater [12–14]. The efficient and safe management of uranium-containing wastewater to prevent secondary environmental contamination and recover valuable uranium resources has also become significantly important for the nuclear energy industry [15–17].
Nevertheless, the recovery of dispersed uranium resources from seawater and uranium-containing wastewater presents a substantial challenge because of low uranium concentrations, excessive co-existing interfering ions, and harsh environmental conditions [18–20]. Contrasted with methods such as liquid–liquid extraction [21,22], ion exchange [23,24], coprecipitation [25,26], evaporation [27] and membrane filtration [28,29], solid-phase adsorption [30–32] is considered as the most advantageous technique for practical applications. Improvements in the adsorbent performance in the solid-phase adsorption method has garnered significant attention [33]. The faster uranium adsorption kinetics of the solid-phase adsorption method can shorten the extraction cycle, thereby reducing the cost of uranium recovery [34]. The extraction process of uranium by adsorbents includes surface adsorption and internal diffusion [35]. The binding of uranyl ions by the functional sites happens in a rapid process, whereas the accessibility of uranyl ions is limit by their diffusion ability within the adsorbents, which limits the uranium adsorption rate [36]. Optimizing adsorbent structures to shorten the diffusion pathway of uranyl ions within the adsorbents has been proven to markedly enhances their uranium adsorption efficiency. Accordingly, numerous adsorbents, including ultrafine fibers and ultrathin membranes with exceptionally short uranium diffusion paths, have been developed to enhance uranium extraction rates. Notable advancements have been achieved by the development of blow-spinning ultrafine oxime-based nanofibers [37], ultrathin oxime-based membrane [38], and polyamidoxime (PAO) nanofibers adsorbent [39]. However, due to their fragile structures, although these adsorbents have realized improved uranium extraction performance, they cannot resistant to harsh forces, including the force during the operation of the adsorbent and the impact of water after being deployed, thus failing to satisfy practical application requirements [40,41].
Block adsorbents with super-size are desirable for practical applications. However, the dense structures of block adsorbents hinder the diffusion of uranyl ions into their interior, leading to a limited adsorption capacity [42]. Recently, a novel strategy to enhance uranium extraction performance of block adsorbents have emerged by introducing interconnected large pores as water transport channels to enhance uranium extraction performance by reducing the mass transfer distance [43–46]. Several methods have been proposed to design adsorbent with water transport channels, such as the use of sturdy frames as supports and ice templating methods [47,48]. Nevertheless, these strategies possess several inherent deficiencies. The sturdy frame lacks functional sites for uranyl ion binding, which consequently constrains the enhancement of adsorption capacity. The ice templating strategy requires complicated and precise operational processes for constructing water transport channels within the adsorbents [49], hence elevating the fabrication costs and posing challenges for the fabrication of large-scale adsorbents. Furthermore, available adsorbents fabricated using the ice templating method also exhibit low mechanical stability, due to their predominant structure of directionally aligned channels, which lack stable vertical support [50]. Therefore, developing a new method for fabricating adsorbents with enhanced uranyl ion diffusion and high mechanical stability is crucial.
Attributing to the excellent binding affinity of the amidoxime (AO) group to uranyl ions, AO-based adsorbents have been widely studied for uranium extraction from seawater and wastewater [51–54]. In this work, we propose a facile method to fabricate PAO sponge adsorbent with super-sized water transport channels (SWTC-PAO sponge) and stable structure by utilizing sugar particles of different sizes as sacrificial templates for recovery of dispersed uranium (Figure 1). The fabrication process does not require energy-intensive equipment and complex preparation processes, which is preferable for industrial mass production. Compared with the reported block AO-based adsorbents, the SWTC-PAO sponge showed an overall improved performance in ease of fabrication, mechanical stability, and adsorption performance. Owing to these advantages, the SWTC-PAO sponge is promising for uranium recovery from seawater and uranium-containing wastewater at high efficiency. Furthermore, the metal ions concentrations in the SWTC-PAO sponge-treated wastewater are lower than the standard for drinking water quality set by the World Health Organization (WHO). These results prove that the SWTC-PAO sponge is a highly promising candidate for recovery of dispersed uranium and remediation of environmental uranium contamination.
 |
Figure 1 Schematic of the huge block adsorbent SWTC-PAO sponge with super-sized water transport channels for ultrafast and high-capacity recovery of dispersed uranium. The limitations of the other relevant adsorbents have also been shown. |
MATERIALS AND METHODS
Materials
Polyacrylonitrile (PAN, Mw = 150,000, Macklin), hydroxylamine hydrochloride (NH2OH·HCl, 99%, Macklin), sodium hydroxide (NaOH, 99.5%, Aladdin), sodium carbonate (Na2CO3, 99.8%, Macklin), N,N-dimethylformamide (DMF, GC, Macklin), N,N-dimethylacetamide (DMAC, GC, Macklin), sugar particles (Taikoo), methyl alcohol (CH4O, 99%, Macklin), sodium chloride (NaCl, 99.5%, Aldrich), sodium bicarbonate (NaHCO3, 99.8%, Macklin), uranyl nitrate hexahydrate (UO2(NO3)2·6H2O, 99%, Macklin), hydrogen peroxide (H2O2, 30% in water, Macklin), and muriatic acid (HCl, 99.9%, Xilong Scientific) were procured economically. All reagents were used as received without further purification.
Synthesis of HV-PAO solution and PAO solution
A high viscosity PAO (HV-PAO) solution was prepared by referring to a previously reported method with some modifications. Initially, 12 g of NH2OH·HCl was added to a round-bottom flask containing a mixed solution of 200 mL DMAC and DMF (VDMAC:VDMF = 6:4). Once the chemical reagent was fully dissolved, 3 g of NaOH and 3.9 g of Na2CO3 were added, and the flask was placed in a 45 °C oil bath under mechanical stirring. After approximately 3 h, the pH of the mixed solution reached neutrality, and 10 g of PAN was gradually added while stirring. Following a 20 h reaction at 75 °C, a viscous yellow solution was formed, which was then centrifuged at 10,000 r/min for 40 min. The supernatant was separated to obtain the HV-PAO solution. PAO solution was also prepared by only using DMF as the solvent, the other procedures were the same as the method for fabricating the HV-PAO solution.
Fabrication of SWTC-PAO sponge and PAO membrane
The HV-PAO solution was thoroughly mixed with sugar particles at different mass ratios (mHV-PAO solution:msugar particles = 1:3, 1:3.5, 1:4.0, 1:4.5, 1:5) for 2 h and treated by anhydrous methanol to induce phase separation at room temperature. After soaking in anhydrous methanol for 10 h, the phase separated mixture was immersed in ultrapure water to remove the sugar particles, and the SWTC-PAO sponge with closed cavities was obtained. After further treatment with water pressure generated by a diaphragm, an SWTC-PAO sponge with interconnected channels was formed and used for subsequent studies after drying. To fabricate the PAO membrane, the PAO solution was spread onto a glass mold and soaked in deionized water for 1 h. After being dried at 60 °C, PAO membrane was obtained and used for the following study.
Characterization of the materials
Scanning electron microscopy (SEM, S-4800, HITACHI, Japan) and energy-dispersive X-ray spectroscopy (EDS, Nano XFlash Detector 5030, Bruker, Germany) were used to visualize the micromorphology of the adsorbent and determine the elemental distribution, respectively. Digital viscometer (NDJ-5S, Shanghai HengPing Instrument and Meter Factory, China) was used to measure the viscosities of the HV-PAO and PAO solutions. The membrane flux tester (SF-SA, Hangzhou Saifei Membrane Separation Technology Co., Ltd, China) was used to measure the water flux of adsorbents. Fourier transform infrared spectroscopy (FT-IR, LR 64912C, Frontier, PerkinElmer, USA) was used to determine the chemical composition of the materials. UV–Vis spectrophotometer (UV-Vis, UV1800PC, AuCy Instrument, China) was used to measure the uranium concentration in the uranium-spiked simulated seawater. X-ray photoelectron spectroscopy (XPS, Thermo escalab 250Xi Thermo, USA) was used to analyze the surface elemental composition and binding energy of the materials. The water contact angle measuring instrument (JC2000D, Shanghai Zhongchen Digital Technic Apparatus CO., LTD, China) was used to analyze the hydrophilicity of the materials. N2 adsorption/desorption porosimetry (ASAP 2460, Micromeritics, USA) and mercury intrusion porosimetry (MIP, AutoPore V9620, Micromeritics, USA) were used to measure the specific surface areas and pore size distribution of the materials. Inductively coupled plasma-mass spectrometry (ICP-MS, iCAP RQplus, Thermo Fisher Scientific, USA) was used to determine the concentrations of metal ions in natural seawater and uranium-containing wastewater.
Simulation of the flow resistance of adsorbent
The internal fluid pressure inside the adsorbents with different channel sizes was simulated using the fluid dynamics module in the COMSOL multi-physics field finite element simulation software. The water channels inside the adsorbent were modeled as densely packed hexagonal prisms with water channel sizes of 10, 100, 200, and 1000 μm and channel wall thickness of 1 and 10 μm to simulate available adsorbent structures. The total thickness of the adsorbent was set at 1000 μm. The walls of the channels were set to no-slip condition. Laminar water flow was introduced from the upper side of the adsorbent with a flow rate of 40 cm/s and flowed out from the lower side by setting the pressure at the outlet to 0 Pa. Steady-state simulations based on the above setup yielded the internal water pressure distribution within the channels.
Measurements of the water flux of adsorbent
SWTC-PAO sponge with a thickness of 2 mm was installed in the dead-end flow channel with an active area of 22.9 cm2 and water flow with an initial pressure of 0.1 to 2 bar was flowed through the adsorbent. The water flux of the absorbent was calculated using Equation (1).
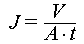 (1)
(1)
where J (L h−1 m−2) represents the water flux, A (m−2) represents the active area, t (h) represents the test time, and V (L) represents the volume of infiltrated water during the test time.
Uranium extraction capacity assay in uranium-spiked simulated seawater
The simulated seawater was prepared by dissolving NaCl (438.607 mM) and NaHCO3 (2.297 mM) in ultrapure water. SWTC-PAO sponge was pretreated with NaOH solution (40 mM) at 60 °C and further treated with simulated seawater to induce the salt-shrinkage of the adsorbent. Subsequently, the material was washed with ultrapure water and used for uranium adsorption experiments. The optimal pH for uranium adsorption was determined using 8 ppm uranium-spiked simulated seawater at pH 4, 5, 6, 7, 8, and 9. Alkali-treated SWTC-PAO sponge with an initial dry weight of 10 mg was loaded into the extraction unit designed in this study, and 2 L of uranium-spiked simulated seawater was flowed through the adsorbent at a flow rate of 800 mL/min at 35 °C. Aqueous samples were collected every 2 h until adsorption equilibrium was reached, and the uranium concentration was analyzed using UV–Vis spectrophotometry. The uranium extraction capacity of the adsorbent was calculated using Equation (2).
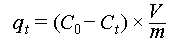 (2)
(2)
where qt (mg g−1) represents the uranium extraction capacity after contact time t, C0 (mg L−1) represents the initial uranium concentration, Ct (mg L−1) represents the uranium concentration at time t, V (L) represents the volume of the used uranium solution, and m (g) represents the weight of the used adsorbent.
The uranium adsorption kinetics of the SWTC-PAO sponge were determined using uranium-spiked simulated seawater with uranium concentrations of 2, 4, 8, and 16 ppm at a pH of 6. The dosage of the adsorbent, the interval of aqueous samples collection, and the method for measuring the uranium concentration were consistent with previous experiments. The experimental adsorption data were fitted to pseudo-first- and pseudo-second-order kinetic models using Equations (3) and (4).
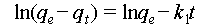 (3)
(3)
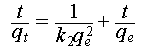 (4)
(4)
where qt (mg g−1) and qe (mg g−1) represent the amounts of uranium adsorbed by the material after adsorption for t hour and at equilibrium, respectively, and k1 (min−1) and k2 (g mg−1 min−1) represent the rate constants for the pseudo-first-and second-order kinetic models, respectively.
The isothermal adsorption curve was determined using uranium-spiked simulated seawater with uranium concentrations of 2, 4, 8, 16, 32, and 64 ppm at pH 6. The theoretical maximum uranium extraction capacity of the sorbent was calculated using Equations (5) and (6).
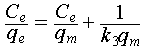 (5)
(5)
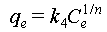 (6)
(6)
where qe (mg g−1) and Ce (ppm) represent the capacity of uranium adsorbed by the material and the concentration of uranium at equilibrium, respectively, qm (mg g−1) represents the saturated adsorption capacity, and k3 (L mg−1) and k4 (L g−1) represent the equilibrium constants related to the binding strength.
Reusability assay
The elution solution composed of 1.0 M Na2CO3 and 0.1 M H2O2 was used to elute the bound uranium at 25 °C. Eluates were collected every 30 s until desorption reached equilibrium and the uranium concentration was analyzed by UV–Vis spectrophotometry. The uranium elution efficiency (EE, %) was calculated using Equation (7).
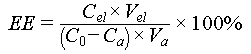 (7)
(7)
where Cel (mg L−1) represents the uranium content in the eluate, Vel (L) represents the used volume of elution solution, Ca (mg L−1) represents the uranium concentration in the uranium-spiked seawater after uranium adsorption, C0 (mg L−1) represents the initial uranium concentration of the uranium-spiked seawater, and Va (L) represents the volume of the uranium-spiked seawater used for adsorption.
Uranium extraction from natural seawater
Natural seawater was obtained from the South China Sea near Haikou city, Hainan province, China, and filtered using a 0.45 μm filter membrane to remove marine bacteria and suspended particles for determining the uranium extraction capacity of the SWTC-PAO sponge. Alkali-treated SWTC-PAO sponge with an initial dry weight of 10 mg was loaded into the extraction unit and 100 L of natural seawater was flowed through the adsorbent at a flow rate of 800 mL/min at 25 °C. Seawater samples were collected every 3 days until the extraction process reached equilibrium and the uranium extraction capacity was determined using ICP-MS.
Uranium recovery from uranium-containing wastewater
The uranium-containing wastewater with a pH of 6 was collected during the daily uranium adsorption capacity assay trails to evaluate the uranium recovery ability in uranium-containing wastewater. Alkali-treated SWTC-PAO sponge with an initial weight of 1.75 g was loaded into the extraction unit and 73 L of uranium-containing wastewater was flowed through the adsorbent at a flow rate of 800 mL/min at 25 °C. Wastewater samples were collected every 1 h until reaching adsorption equilibrium and the uranium concentration was analyzed by ICP-MS.
RESULTS AND DISCUSSION
Fabrication and characterization of adsorbent
The material designed in this study was fabricated using a scalable fabrication strategy that integrates phase separation and template methods, to obtain stability structure huge block adsorbent with super-sized water transport channels for the recovery of dispersed uranium resources (Figure 2A). In detail, the HV-PAO solution was prepared through the amidoximation of PAN in DMF with the supply of DMAC, which facilitates the incorporation of additional Na+ to form ionic bonds between AO groups and endows the prepared PAO solution with a higher viscosity of 538 mPa·s than that of the PAO solution (32 mPa·s) fabricated without DMAC (Figure S1). Sugar particles with particle sizes ranging from 200 to 900 μm were used as sacrificial templates and uniformly mixed with the HV-PAO solution (Figure S2). The mixture was further treated with methanol to induce phase separation, and the sugar particles were dissolved in ultrapure water to form an SWTC-PAO sponge with closed cavity walls, which was subsequently broken to generate an SWTC-PAO sponge with interconnected 3D super-sized water transport channels (Figure S3). To optimize the channel structure, the weight ratio between the HV-PAO solution and sugar particles was adjusted (Figure S4). With the mass ratio of 1:4.5 between the HV-PAO solution and sugar particles, the prepared adsorbent exhibited a distinct channel structure and desirable uranium adsorption performance. A higher mass ratio of sugar particles led to a decrease in material stability, whereas a lower mass ratio resulted in a reduction in the channel structure, leading to a decline in the uranium adsorption capacity. Benefitting from the easy preparation process, this strategy can be used to fabricate large-sized adsorbents with abundant pore structures (Figures 2B and S5).
 |
Figure 2 Fabrication diagram and characterization of SWTC-PAO sponge. (A) Schematic for the fabrication of SWTC-PAO sponge. (B) Macroscopic and microscopic photos of SWTC-PAO sponge. (C) Detail channel structures of SWTC-PAO sponge observed by SEM. (D) Pore size distribution of PAO membrane and SWTC-PAO sponge obtained by N2 adsorption/desorption isotherm and MIP analyses. (E) Mechanical property of SWTC-PAO sponge. |
The prepared material was characterized to confirm the successful fabrication of the SWTC-PAO sponge. The FT-IR spectra of SWTC-PAO sponge showed the disappearance of the characteristic peak for –C≡N (2245 cm−1) in PAN and the appearance of the characteristic peak for –C=N– (1665 cm−1) in PAO, indicating the successful amidoximation of PAN to PAO (Figure S6). In addition, the characteristic peaks of granulated sugar could not be detected in the FT-IR spectrum of the SWTC-PAO sponge, indicating the complete removal of sugar particles. SEM observation indicated that the interior structure of the SWTC-PAO sponge consisted of interconnected super-sized channels with diameters up to 830 μm and thin walls with thickness ranging from 5 to 10 μm together with the connection points among the thin walls with diameters ranging from 80 to 100 μm (Figure 2C). Previously reported PAO membrane was also fabricated using the methanol-induced phase separation method without applying of the sugar templating method. The pore size distribution and cumulative pore area of the SWTC-PAO sponge and PAO membrane were determined using N2 adsorption/desorption isotherm and MIP analyses (Figures 2D and S7). The results of the MIP analysis indicated that the sugar templating method could effectively introduce large pores with diameters ranging from 10 μm to 830 μm into the SWTC-PAO sponge, contributing to the pore volume of 22.95 cm3 g−1, which was 27 times greater than that for PAO membranes (0.81 cm3 g−1). Additionally, the methanol-induced phase separation process introduced numerous small pores with diameters ranging from 1.5 to 80 nm into the SWTC-PAO sponge with the BET specific surface area of 15.79 m2 g−1, which was 3.07 times that of the PAO membrane (5.15 m2 g−1). The formation of submillimeter and micrometer macropores may facilitate efficient water transport channels for transporting uranyl ions to the adsorption sites within the adsorbent and the formation of nanopores could provide sufficient surface area to display uranium adsorption sites, which both could potentially benefit the uranium adsorption performance of the SWTC-PAO sponge.
Hydrophilicity is another important factor affecting the adsorption performance. Compared to the PAO membrane, the SWTC-PAO sponge exhibited higher hydrophilicity, which was attributed to the heightened surface roughness resulting from its abundant microporous structure (Figure S8). Due to the existence of abundant interconnected thin-wall structure to share the force, SWTC-PAO sponge exhibited high mechanical strength to resistant the pressure as high as 106.7 kPa and could withstand the heavy loads without structural damage (Figure 2E and Figure S9). The high hydrophilicity and mechanical properties of the SWTC-PAO sponge enhance its application in natural environment.
Influence of adsorbent water channel sizes on water transport
The efficient transport of water within the block adsorbent is essential for the high-capacity adsorption of uranium and the channels structure of the adsorbent is the most important factor that determined the water transport ability. Previous studies have reported the construction of water channels in uranium adsorbents using phase separation, ice templating, sturdy frame support and foaming. Hoverer, these methods can only obtain water channels with small sizes and narrow distribution ranges. In contrast to previous reports, the strategy used in this work produced adsorbent with super-sized and widely distributed water channels (Figure 3A and Table S1). The influence of the channel size on the water transport resistance was simulated by calculating the stress distribution of water in the channels. The results showed that, under the water flow impact with a velocity of 40 cm/s, the maximum stress of water in the adsorbent with channel sizes of 10, 100, 200, and 1000 μm was 46000, 600, 150 and 20 Pa, respectively, indicating that the increase in channel size could substantially reduce the resistance for water transport (Figure 3B–3E). Dead-end water flux test was employed to determine the water transport ability of the SWTC-PAO sponge and excellent water transport ability with high water flux of 6459 L h−1 m−2 and 2516 L h−1 m−2 at the water pressure of 1.0 bar and 0.2 bar, respectively, were realized (Figure S10). These results confirmed the contribution of the large channel size to the efficient transport of water within the adsorbent.
 |
Figure 3 Simulation of the channel size on the water transport ability. (A) Channel size distribution of uranium adsorbents prepared using different methods as reported in literature. Corresponding references are shown in Table S1. (B–E) Simulated stress distributions of water in channels of different sizes under the water flow impact with a velocity of 40 cm/s. |
Uranium extraction from uranium-spiked simulated seawater and natural seawater
To evaluate the uranium adsorption performance of the adsorbent, extraction equipment was designed (Figure 4A). The adsorbent was loaded into the extraction unit and the uranium-containing solution flowed through the extraction unit to allow the adsorption of uranium by the adsorbent. The effect of environmental pH on the uranium adsorption performance of the SWTC-PAO sponge was analyzed in a pH range of 3–8 in 8 ppm uranium-spiked simulated seawater (Figure 4B and Figure S11). The results showed that the SWTC-PAO sponge exhibited the highest uranium adsorption capacity at pH 6 with the equilibrium adsorption capacity of 733.8 mg g−1 and a desirable uranium adsorption equilibrium capacity of 532.4 mg g−1 at pH 8. Within the pH range of 5–8, the SWTC-PAO sponge maintained a high uranium adsorption ability, indicating that the adsorbent might be applied in broad environments. Attributed to the strong electrostatic repulsion between the protonated SWTC-PAO sponge and positively charged uranyl species, the uranium adsorption capacity was significantly reduced in the pH range of 3–4 [55]. The uranium adsorption kinetics of SWTC-PAO sponge demonstrated a rapid uranium extraction rate in uranium-spike simulated seawater, achieving high uranium adsorption capacities of 278.3 mg g−1, 437.6 mg g−1, 711.9 mg g−1 and 911.7 mg g−1 in 2, 4, 8, and 16 ppm uranium-spiked simulated seawater, respectively, within 16 h (Figure 4C and Table S2). Notably, the adsorption capacity reached half of the equilibrium adsorption capacity within 4 h. The fitting results of the adsorption kinetics curve were in better agreement with the pseudo-second-order kinetic model, confirming that uranyl ions were primarily chemically adsorbed by the SWTC-PAO sponge. Adsorption isotherm experiments were conducted to assess the theoretical maximum uranium adsorption capacity of the SWTC-PAO sponge. The adsorption isotherm of the SWTC-PAO sponge fitted well with the Langmuir model, proving the monolayer chemical adsorption behavior of the SWTC-PAO sponge for uranium (Figure S12 and Table S3). The theoretical maximum uranium adsorption equilibrium capacity of the SWTC-PAO sponge was calculated to be approximately 1390 mg g−1. In addition, the uranium adsorption capacity of the SWTC-PAO sponge was determined by directly placing it into simulated seawater rather than flowing the simulated seawater through it. The results showed that only an 8.5% decrease in uranium adsorption capacity, indicating that the SWTC-PAO sponge could also be used by directly placing it in the ocean (Figure S13). The high uranium adsorption capacity and rapid adsorption rate were attributed to the interconnected channels that facilitated the rapid transportation of uranyl ions to the uranium adsorption sites and the presence of ultrathin channel wall structures that presented more accessible active sites for uranium adsorption. The SWTC-PAO sponge remained intact structure after being used for uranium adsorption in simulated seawater (Figure 4D). The reusability of the material was evaluated, and the results showed that the SWTC-PAO sponge could be regenerated in situ within the extraction unit. The elution solution comprising Na2CO3 and H2O2 was used to elute the bound uranium, which was based on the mechanism that the used elution solution can form highly stable uranyl peroxo-carbonato complexes [UO2(O2)(CO3)24−] by competing with bound uranyl ions [56]. More than 95% of the bound uranium could be effectively eluted by the elution solution within 2 min (Figure S14). After 10 reuse cycles, the adsorption capacity of the SWTC-PAO sponge decreased to 77.4% of its initial capacity with an average decrease of only 2.8% per cycle (Figure 4E). Meanwhile, after 10 reuse cycles, more than 94% of the uranium bound by the adsorbent could be eluted. SWTC-PAO sponge realized an excellent uranium extraction performance of 8.4 mg g−1 after 15 d and a high adsorption rate of 0.56 mg g−1 d−1 in natural seawater (Figure 4F and Table S5). Furthermore, the SWTC-PAO sponge exhibited high selectivity for uranium, achieving a uranium–vanadium adsorption ratio of 1.49 in natural seawater containing diverse metal ions (Figure S15 and Table S4). With the extension of the extraction time to 30 days, the uranium extraction capacity reached 9.1 mg g−1. More importantly, the SWTC-PAO sponge demonstrated structural integrity after 10 cycles of uranium adsorption-desorption and after 30 of uranium extraction from natural seawater (Figure S16).
 |
Figure 4 Uranium extraction performance in uranium-spiked simulated seawater and natural seawater. (A) Structural diagram of uranium extraction unit for evaluating uranium adsorption performance. SWTC-PAO sponge is fixed inside the uranium extraction unit by placing it onto the porous support plate. (B) Uranium extraction capacity of SWTC-PAO sponge under different pH. (C) Uranium adsorption kinetics of SWTC-PAO sponge under different initial uranium concentrations in simulated seawater. (D) Morphology of SWTC-PAO sponge after being used for uranium adsorption in simulated seawater. (E) Reusability of SWTC-PAO sponge. (F) Extraction kinetics of SWTC-PAO sponge for uranium in filtered natural seawater. |
The uranium-loaded SWTC-PAO sponge was characterized. The SEM and corresponding EDS mapping analysis showed that uranium together with nitrogen, oxygen, and carbon were uniformly distributed in the uranium-loaded SWTC-PAO sponge (Figure S17). The FT-IR spectra of uranium-loaded SWTC-PAO sponge presented a peak at 917 cm−1, belonged to the O=U=O bonds (Figure S18) [57]. The X-ray photoelectron spectroscopy (XPS) analysis also proved the existence of characteristic peaks for the U 4f orbitals of uranyl ions (Figure S19). Compared to the narrow scan XPS spectrum of UO2(NO3)2·6H2O, two additional peaks were observed at 381.48 and 392.28 eV for uranium-loaded SWTC-PAO sponge (Figure S20). As for the narrow scan XPS spectrum of O 1s, an extra peak at 533.04 eV was detected in uranium-loaded SWTC-PAO sponge compared with that of UO2(NO3)2·6H2O and SWTC-PAO sponge (Figure S21). The decrease in the binding energy of uranium and the increase in the binding energy of oxygen confirmed the formation of coordination bonds between uranium and the AO group in the SWTC-PAO sponge (Figure S16). The van der Waals surface diagram of the electrostatic potential (ESP), revealing that oxygen atoms from AO groups exhibited a negative electrostatic potential, facilitating the binding of positive electrostatic potential uranyl ions (Figure S22). These results indicate that SWTC-PAO exhibits promising uranium adsorption ability in the presence of the AO group.
Uranium recovery from uranium-containing wastewater
To evaluate the ability of the SWTC-PAO sponge to recover uranium from wastewater, a simple route was designed to treat 73 L of uranium-containing wastewater at pH 6. The uranium extraction device for uranium recovery from wastewater consisted of four parts, including a liquid storage tank, an extraction unit, a pumping device, and a circulation pipeline (Figure 5A). The extraction unit was filled with three layers of SWTC-PAO sponge with a total initial dry weight of 1.75 g. After the extraction process, the color of the SWTC-PAO sponge changed from light yellow to orange-red, indicating the binding of uranium (Figure 5B). After the elution of uranium, the SWTC-PAO sponge remained light yellow color because the other metal ions were also adsorbed from the wastewater and could not be efficiently eluted by the elution solution [56]. Inductively coupled plasma mass spectrometry (ICP-MS) analysis revealed that the initial wastewater contained a variety of metal ions, with the uranium content was 5.22 ppm, approximately 1600 times that in natural seawater (Figure 5C). After 24 h of extraction, the concentrations of all metal ions in the wastewater decreased significantly and were lower than the standard set for drinking water quality by the WHO (Table S6). Specifically, 99.59% of the uranium was extracted, and the remaining uranium concentration decreased to 21.00 ppb (Figure 5D). The uranium adsorption capacity of the SWTC-PAO sponge was 216.87 mg g−1. Afterwards, the uranium in the eluate was purified to obtain UO2(NO3)2·6H2O (Figure 5E). In detail, the eluent was treated with 10 M HNO3 to remove the CO32− group, and uranium was further precipitated using 10 M NaOH. The precipitate was then dissolved in 10 M HNO3 and extracted with tributyl phosphate (TBP) solution to form stable uranium-TBP complexes for uranium purification [58]. The extracted uranium was precipitated with NH3·H2O solution to obtain ((NH4)2U2O7), which was subsequently dissolved in 10 M HNO3 and heated to remove H2O and NH4+. Finally, a product weighing 726 mg was obtained via evaporation crystallization process, accounting for 90.68% of the uranium in the initially used 73 L of uranium-containing wastewater. XRD analysis confirmed that the recovered product existed in the form of UO2(NO3)2·6H2O (Figure 5F). These results demonstrated the applicability of the SWTC-PAO sponge for uranium recovery from uranium-containing wastewater.
 |
Figure 5 Uranium recovery from uranium-containing wastewater. (A) Schematic of the uranium recovery device for treating uranium-containing wastewater. (B) Images of SWTC-PAO sponge loaded in the extraction unit. (C) Metal ion concentrations in the wastewater before and after uranium recovery. (D) Extraction kinetics of SWTC-PAO sponge in wastewater. The change curve of uranium concentration is shown. (E) Process for separating and purifying uranium from the eluate. (F) XRD patterns of UO2(NO3)2·6H2O and the product recovered from uranium-containing wastewater. |
CONCLUSIONS
The recovery of uranium from seawater and uranium-containing wastewater is importance for fueling the nuclear energy industry and treating environmental uranium contamination. Facing the shortcoming that hinder the improvement of the uranium recovery performance of adsorbents, this study employed a combined facile phase separation method and templating method to fabricate huge block SWTC-PAO sponge with interconnected super-sized water transport channels for efficient transport of uranium into the interior of the adsorbent. Meanwhile, the ultrathin channel walls formed in the adsorbent significantly improved the number of accessible functional sites for uranium binding. Furthermore, the abundant interconnected ultrathin channel walls could efficiently share the water impact force and provide the adsorbent with excellent mechanical stability hence broaden the environment for the application of this adsorbent. As a result, the SWTC-PAO sponge exhibited considerable potential for application in both basic natural seawater and acidic uranium-containing wastewater. In natural seawater, the SWTC-PAO sponge realized a high uranium extraction capacity of 9.1 mg g−1 with rapid uranium extraction rate. In uranium-containing wastewater, the SWTC-PAO sponge removed 99.59% of the uranium and improved the quality of the treated wastewater to meet the drinking water quality standard set by the WHO. Considering its excellent uranium extraction performance, high structural stability, and simple preparation process, the SWTC-PAO sponge is a promising candidate for recovering dispersed uranium resources and treating uranium contamination under diverse conditions.
Data availability
The original data are available from corresponding authors upon reasonable request.
Funding
This work was supported by the Nuclear Technology R&D Program, the National Natural Science Foundation of China (U2167220, 22327807 and U23A20104), the specific research fund of the Innovation Platform for Academicians of Hainan Province (YSPTZX202214 and YSPTZX202316), the National Key Research and Development Program of China (2023YFC2809000), the Innovation Fund for Scientific and Technological Personnel of Hainan Province (KJRC2023B01), and the Hainan Province Science and Technology Special Fund (ZDYF2024SHFZ066).
Author contributions
Y.Y., N.W. and J.Z. conceived the concept and designed the research. J.Z., M.C., S.Z., H.W., Y.J., Y.M. and L.F. conducted the experiments. J.Z., Y.Y. and N.W. wrote the manuscript. Y.Y., N.W. and J.Z. discussed the results and contributed to the concept development.
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
Supplementary file provided by the authors. Access Supplementary Material
The supporting information is available online at https://doi.org/10.1360/nso/20240020. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
References
- Parsons J, Buongiorno J, Corradini M, et al. A fresh look at nuclear energy. Science 2019; 363: 105. [Article] [Google Scholar]
- Wang C, Helal AS, Wang Z, et al. Uranium in situ electrolytic deposition with a reusable functional graphene‐foam electrode. Adv Mater 2021; 33: 2102633. [Article] [CrossRef] [PubMed] [Google Scholar]
- Mazzucchi N. Nuclear power can help the democratic world achieve energy independence. Nature 2022; 606: 841 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Demski C, Poortinga W, Whitmarsh L, et al. National context is a key determinant of energy security concerns across Europe. Nat Energy 2018; 3: 882-888. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Yang H, Liu X, Hao M, et al. Functionalized iron–nitrogen–carbon electrocatalyst provides a reversible electron transfer platform for efficient uranium extraction from seawater. Adv Mater 2021; 33: 2106621. [Article] [CrossRef] [Google Scholar]
- Costa Peluzo BMT, Kraka E. Uranium: The nuclear fuel cycle and beyond. Int J Mol Sci 2022; 23: 4655. [Article] [Google Scholar]
- Tsouris C. Uranium extraction: Fuel from seawater. Nat Energy 2017; 2: 17022 [CrossRef] [Google Scholar]
- Wang Z, Meng Q, Ma R, et al. Constructing an ion pathway for uranium extraction from seawater. Chem 2020; 6: 1683-1691. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Abney CW, Mayes RT, Saito T, et al. Materials for the recovery of uranium from seawater. Chem Rev 2017; 117: 13935-14013. [Article] [CrossRef] [PubMed] [Google Scholar]
- Zhang S, Chen L, Qu Z, et al. Confining Ti-oxo clusters in covalent organic framework micropores for photocatalytic reduction of the dominant uranium species in seawater. Chem 2023; 9: 3172-3184. [Article] [Google Scholar]
- Zhang H, Liu W, Li A, et al. Three mechanisms in one material: Uranium capture by a polyoxometalate–organic framework through combined complexation, chemical reduction, and photocatalytic reduction. Angew Chem Int Ed 2019; 58: 16110-16114. [Article] [Google Scholar]
- Lin T, Chen T, Jiao C, et al. Ion pair sites for efficient electrochemical extraction of uranium in real nuclear wastewater. Nat Commun 2024; 15: 4149. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Sharma M, Dhiware P, Laddha H, et al. Harnessing magnetically separable iron based adsorbents for enhanced uranium adsorption. Coord Chem Rev 2024; 508: 215766. [Article] [Google Scholar]
- Zhang H, Li A, Li K, et al. Ultrafiltration separation of Am(VI)-polyoxometalate from lanthanides. Nature 2023; 616: 482-487. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Zhang Q, Luo F. Fabricating boron-functionalized covalent organic framework with remarkable potential in handling cationic, anionic, and gaseous nuclear wastes. Adv Funct Mater 2024; 34: 2401775 [CrossRef] [Google Scholar]
- Dong Z, Gao D, Li Z, et al. Harvesting the vibration energy of CdS for high-efficient piezo-photocatalysis removal of U(VI): Roles of shape dependent and piezoelectric polarization. Energy Environ Mater 2024; 7: e12705 [CrossRef] [Google Scholar]
- Liu Y, Guo XQ, Li SW, et al. Discharge of treated Fukushima nuclear accident contaminated water: Macroscopic and microscopic simulations. Natl Sci Rev 2021; 9: nwab209. [Article] [Google Scholar]
- Zhang F, Dong H, Li Y, et al. In situ metal‐oxygen‐hydrogen modified B-TiO2 @Co2 P-X S-scheme heterojunction effectively enhanced charge separation for photo‐assisted uranium reduction. Adv Sci 2024; 11: 2305439. [Article] [Google Scholar]
- Feng L, Wang H, Feng T, et al. In situ synthesis of uranyl‐imprinted nanocage for selective uranium recovery from seawater. Angew Chem Int Ed 2022; 61: e202101015. [Article] [CrossRef] [Google Scholar]
- Yuan Y, Yu Q, Cao M, et al. Selective extraction of uranium from seawater with biofouling-resistant polymeric peptide. Nat Sustain 2021; 4: 708-714. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Hadadian M, Mallah MH, Moosavian MA, et al. Separation of uranium (VI) using dispersive liquid-liquid extraction from leach liquor. Prog Nucl Energy 2016; 90: 212-218. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Boyarintsev AV, Perevalov SA, Stepanov SI, et al. Liquid–liquid extraction of neptunium(VI) and neptunium(V) from carbonate solutions by methyltrioctylammonium carbonate in toluene. J Radioanal Nucl Chem 2021; 327: 385-393. [Article] [Google Scholar]
- Phillips DH, Gu B, Watson DB, et al. Uranium removal from contaminated groundwater by synthetic resins. Water Res 2008; 42: 260-268. [Article] [CrossRef] [PubMed] [Google Scholar]
- Feng ML, Sarma D, Gao YJ, et al. Efficient removal of [UO2]2+, Cs+, and Sr2+ ions by radiation-resistant gallium thioantimonates. J Am Chem Soc 2018; 140: 11133-11140. [Article] [Google Scholar]
- Dickinson M, Scott TB. The application of zero-valent iron nanoparticles for the remediation of a uranium-contaminated waste effluent. J Hazard Mater 2010; 178: 171-179. [Article] [CrossRef] [PubMed] [Google Scholar]
- Li ZJ, Wang L, Yuan LY, et al. Efficient removal of uranium from aqueous solution by zero-valent iron nanoparticle and its graphene composite. J Hazard Mater 2015; 290: 26-33. [Article] [CrossRef] [PubMed] [Google Scholar]
- Dmitriev MS, Kolyaskin AD, Krasnokutskiip RA, et al. Dewatering of salt melts of radioactive wastes from NPP by induction heating. At Energy 2014; 117: 40-43. [Article] [Google Scholar]
- Zhang J, Cui J, Eslava S. Oxygen evolution catalysts at transition metal oxide photoanodes: Their differing roles for solar water splitting. Adv Energy Mater 2021; 11: 2003111. [Article] [CrossRef] [Google Scholar]
- Favre-Réguillon A, Lebuzit G, Murat D, et al. Selective removal of dissolved uranium in drinking water by nanofiltration. Water Res 2008; 42: 1160-1166 [CrossRef] [PubMed] [Google Scholar]
- Kaushik A, Marvaniya K, Kulkarni Y, et al. Large-area self-standing thin film of porous hydrogen-bonded organic framework for efficient uranium extraction from seawater. Chem 2022; 8: 2749-2765. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Yu Q, Yuan Y, Feng L, et al. Spidroin‐inspired, high‐strength, loofah‐shaped protein fiber for capturing uranium from seawater. Angew Chem Int Ed 2020; 59: 15997-16001. [Article] [Google Scholar]
- Qu Z, Leng R, Wang S, et al. Nanomaterials derived from metal–organic frameworks and their applications for pollutants removal. Rev Environ Contam Toxicol 2024; 262: 1210 [Google Scholar]
- Xu X, Zhang H, Ao J, et al. 3D hierarchical porous amidoxime fibers speed up uranium extraction from seawater. Energy Environ Sci 2019; 12: 1979-1988. [Article] [CrossRef] [Google Scholar]
- Liu T, Zhang R, Chen M, et al. Vertically aligned polyamidoxime/graphene oxide hybrid sheets’ membrane for ultrafast and selective extraction of uranium from seawater. Adv Funct Mater 2022; 32: 2111049. [Article] [CrossRef] [Google Scholar]
- Yuan Y, Yang Y, Ma X, et al. Molecularly imprinted porous aromatic frameworks and their composite components for selective extraction of uranium ions. Adv Mater 2018; 30: 1706507. [Article] [CrossRef] [Google Scholar]
- Yang L, Xiao H, Qian Y, et al. Bioinspired hierarchical porous membrane for efficient uranium extraction from seawater. Nat Sustain 2022; 5: 71-80. [Article] [Google Scholar]
- Yuan Y, Zhao S, Wen J, et al. Rational design of porous nanofiber adsorbent by blow‐spinning with ultrahigh uranium recovery capacity from seawater. Adv Funct Mater 2019; 29: 1805380. [Article] [CrossRef] [Google Scholar]
- Yan B, Ma C, Gao J, et al. An ion‐crosslinked supramolecular hydrogel for ultrahigh and fast uranium recovery from seawater. Adv Mater 2020; 32: 1906615. [Article] [CrossRef] [Google Scholar]
- Xu X, Yue Y, Cai D, et al. Aqueous solution blow spinning of seawater‐stable polyamidoxime nanofibers from water‐soluble precursor for uranium extraction from seawater. Small Methods 2020; 4: 2000558. [Article] [CrossRef] [Google Scholar]
- Shi S, Qian Y, Mei P, et al. Robust flexible poly(amidoxime) porous network membranes for highly efficient uranium extraction from seawater. Nano Energy 2020; 71: 104629. [Article] [CrossRef] [Google Scholar]
- Islas AL, Schober CM. Predicting rogue waves in random oceanic sea states. Phys Fluids 2005; 17: 031701. [Article] [CrossRef] [MathSciNet] [PubMed] [Google Scholar]
- Sun Q, Aguila B, Earl LD, et al. Covalent organic frameworks as a decorating platform for utilization and affinity enhancement of chelating sites for radionuclide sequestration. Adv Mater 2018; 30: 1705479. [Article] [CrossRef] [PubMed] [Google Scholar]
- Li D, Liao Y, Chen Z, et al. A 3D hierarchical porous adsorbent constructed by cryo-polymerization for ultrafast uranium harvesting from seawater. J Mater Chem A 2023; 11: 10384-10395. [Article] [Google Scholar]
- Zhang X, Li D, Cui C, et al. Alginate-based supermacroporous hydrogels fabricated by cryo-polymerization for uranium extraction from seawater. Polym Chem 2023; 14: 2902-2915. [Article] [Google Scholar]
- Xu Y, Zhu J, Zhang H, et al. Biomimetic porous cellular foam with space thermal domains for efficient uranium extraction from seawater. J Mater Chem A 2023; 11: 11264-11271. [Article] [Google Scholar]
- Wu Y, Xie Y, Liu X, et al. Functional nanomaterials for selective uranium recovery from seawater: Material design, extraction properties and mechanisms. Coordin Chem Rev 2023; 483: 215097 [CrossRef] [Google Scholar]
- Wang Y, Zhang Y, Li Q, et al. Amidoximated cellulose fiber membrane for uranium extraction from simulated seawater. Carbohydr Polym 2020; 245: 116627. [Article] [Google Scholar]
- Wang Y, Cao M, Peng Q, et al. Polyamidoxime-loaded biochar sphere with high water permeability for fast and effective recovery of uranium from seawater. J Water Process Eng 2023; 55: 104205. [Article] [CrossRef] [Google Scholar]
- Deville S. Wood-like polymeric materials by ice templating. Natl Sci Rev 2018; 6: 184-185 [Google Scholar]
- Zhang X, Yang X, Rong Q, et al. Enrichment and separation of radionuclides by organic polymer materials: A review. Environ Sci Technol 2024; 4: 250-268 [Google Scholar]
- Wang N, Zhao X, Wang J, et al. Accelerated chemical thermodynamics of uranium extraction from seawater by plant‐mimetic transpiration. Adv Sci 2021; 8: 2102250. [Article] [CrossRef] [Google Scholar]
- Ma C, Gao J, Wang D, et al. Sunlight polymerization of poly(amidoxime) hydrogel membrane for enhanced uranium extraction from seawater. Adv Sci 2019; 6: 1900085. [Article] [CrossRef] [Google Scholar]
- Jiao GJ, Ma J, Zhang J, et al. Efficient extraction of uranium from seawater by reticular polyamidoxime-functionalized oriented holocellulose bundles. Carbohydr Polym 2023; 300: 120244. [Article] [Google Scholar]
- Cui WR, Zhang CR, Jiang W, et al. Regenerable and stable sp2 carbon-conjugated covalent organic frameworks for selective detection and extraction of uranium. Nat Commun 2020; 11: 436. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Chen L, Bai Z, Zhu L, et al. Ultrafast and efficient extraction of uranium from seawater using an amidoxime appended metal–organic framework. ACS Appl Mater Interfaces 2017; 9: 32446-32451. [Article] [Google Scholar]
- Pan HB, Liao W, Wai CM, et al. Carbonate–H2O2 leaching for sequestering uranium from seawater. Dalton Trans 2014; 43: 10713-10718. [Article] [CrossRef] [PubMed] [Google Scholar]
- Zhang R, Qiao Q, Liu T, et al. New insights into hydration shells in boosting marine uranium adsorption kinetics. Chem Eng J 2024; 491: 151995. [Article] [CrossRef] [Google Scholar]
- Prabhat P, Rao A, Kumar P, et al. Supercritical fluid extraction and purification of uranium from crude sodium diuranate. Hydrometallurgy 2016; 164: 177-183. [Article] [NASA ADS] [CrossRef] [Google Scholar]
All Figures
 |
Figure 1 Schematic of the huge block adsorbent SWTC-PAO sponge with super-sized water transport channels for ultrafast and high-capacity recovery of dispersed uranium. The limitations of the other relevant adsorbents have also been shown. |
| In the text | |
 |
Figure 2 Fabrication diagram and characterization of SWTC-PAO sponge. (A) Schematic for the fabrication of SWTC-PAO sponge. (B) Macroscopic and microscopic photos of SWTC-PAO sponge. (C) Detail channel structures of SWTC-PAO sponge observed by SEM. (D) Pore size distribution of PAO membrane and SWTC-PAO sponge obtained by N2 adsorption/desorption isotherm and MIP analyses. (E) Mechanical property of SWTC-PAO sponge. |
| In the text | |
 |
Figure 3 Simulation of the channel size on the water transport ability. (A) Channel size distribution of uranium adsorbents prepared using different methods as reported in literature. Corresponding references are shown in Table S1. (B–E) Simulated stress distributions of water in channels of different sizes under the water flow impact with a velocity of 40 cm/s. |
| In the text | |
 |
Figure 4 Uranium extraction performance in uranium-spiked simulated seawater and natural seawater. (A) Structural diagram of uranium extraction unit for evaluating uranium adsorption performance. SWTC-PAO sponge is fixed inside the uranium extraction unit by placing it onto the porous support plate. (B) Uranium extraction capacity of SWTC-PAO sponge under different pH. (C) Uranium adsorption kinetics of SWTC-PAO sponge under different initial uranium concentrations in simulated seawater. (D) Morphology of SWTC-PAO sponge after being used for uranium adsorption in simulated seawater. (E) Reusability of SWTC-PAO sponge. (F) Extraction kinetics of SWTC-PAO sponge for uranium in filtered natural seawater. |
| In the text | |
 |
Figure 5 Uranium recovery from uranium-containing wastewater. (A) Schematic of the uranium recovery device for treating uranium-containing wastewater. (B) Images of SWTC-PAO sponge loaded in the extraction unit. (C) Metal ion concentrations in the wastewater before and after uranium recovery. (D) Extraction kinetics of SWTC-PAO sponge in wastewater. The change curve of uranium concentration is shown. (E) Process for separating and purifying uranium from the eluate. (F) XRD patterns of UO2(NO3)2·6H2O and the product recovered from uranium-containing wastewater. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

