| Issue |
Natl Sci Open
Volume 4, Number 5, 2025
|
|
|---|---|---|
| Article Number | 20250030 | |
| Number of page(s) | 13 | |
| Section | Chemistry | |
| DOI | https://doi.org/10.1360/nso/20250030 | |
| Published online | 20 August 2025 | |
RESEARCH ARTICLE
Parallel emulation of visual adaptation and memory towards biochemically mediated motion recognition in a real scene
1
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China
2
Department of Applied Physics, The Hong Kong Polytechnic University, Hong Kong 999077, China
* Corresponding author (email: This email address is being protected from spambots. You need JavaScript enabled to view it.
)
Received:
28
June
2025
Revised:
11
August
2025
Accepted:
13
August
2025
Abstract
Aqueous neuromorphic devices using essential biological mechanisms have recently appeared as promising candidates for high-level neurosynaptic emulation. Towards the important functionality of motion recognition, while conventional solid-state neuromorphic vision sensors have made significant progress, aqueous motion recognition based on biochemical transmission remains challenging. Taking inspiration from biology, here we report an organic photoelectrochemical transistor biosensor capable of parallel emulation of visual adaptation and memory towards biochemically mediated motion recognition in a real scene. Based on the rational design and implementation of photoelectrochemical events, two artificial Magno and Parvo pathways are emulated to produce visual adaptation and memory in aqueous conditions, respectively. Dynamic and static visual information could be correspondingly processed and applied for integrated image filtering in a real scene and recognition of moving objects by artificial neural networks.
Key words: organic transistor / photoelectrochemical biosensor / aqueous neuromorphic devices / motion recognition / real scene
© The Author(s) 2025. Published by Science Press and EDP Sciences.
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Neuromorphic vision sensor is now experiencing a significant increase in research activity [1–4]. Taking inspiration from the biological retina, it aims to mimic the functions and structures of the retina to enable efficient neuromorphic visual perception. Among various functions, motion recognition has proven to be challenging and demands considerable research efforts in sophisticated algorithms and hardware [5–8]. Solid-state vision sensors have made significant progress [9,10] but still could not compete with nature’s success, which may be due to their fundamentally different synaptic transmission. In the biological vision, motion recognition essentially necessitates biomolecular modulation that alters the states of visual synapses in an aqueous environment [11–14]. It emphasizes the importance of biomolecules and signifies that biomolecular modulation is very needed to achieve motion recognition in aqueous conditions akin to those of biological vision, which, however, is ignored by previous efforts.
Recently, neuromorphic devices capable of emulating the biological mechanisms in aqueous media have become promising contenders in the field of neuromorphic engineering [15–22]. These devices are expected to achieve high-level biomimicry and work in close proximity to and even within biological systems because of their biocompatible nature, i.e., to directly communicate with ions and biochemicals in physiological electrolytes [19–22]. Among them, based on the fusion of organic transistors [23–33] and photoelectrochemistry [34–37], we have proposed an organic photoelectrochemical transistor (OPECT) with unique light-biochemical perception in electrolytes [38–43]. With fused photonic-electrical-biochemical transmission, its synaptic resemblance to that of biological retina has been leveraged for exploring artificial visual devices. Druet et al. [44] used an n-type OPECT regulated by photosensitive organic mixed ionic-electronic charge conductors for the construction of an artificial visual synapse. Corrado et al. [45] deployed an azobenzene-based OPECT to construct neuro-hybrid building block mimicking visual pathways. Hu et al. [46] exploited a hydrogel-based OPECT retinomorphic synapse capable of color perception and biomolecule-mediated synaptic plasticity. Recently, Huang et al. [47] explored OPECT multisensory integration by representative visual (light)-gustatory (sour) perception.
Herein, we propose a bioinspired OPECT biosensor with parallel emulation of visual adaptation and memory towards motion recognition in a real scene. As illustrated (Figure 1a), the human visual system employs visual cells to transform, filter, and process incoming visual information based on biomolecule-mediated synaptic transmission [13,14]. Different visual information (such as shape, brightness, and motion) was processed through two parallel pathways (Figure 1b) [48–50], i.e, the Magno pathway processes the dynamic visual information in parasol ganglion cells [51], while the Parvo pathway processes the static information in midget ganglion cells [52]. By this inspiration, we designed a two-pathway neuromorphic OPECT biosensor, consisting of artificial Mango and Parvo pathways, to emulate the biological visual pathways (Figure 1c). The device operated upon photogating of a poly (3,4-ethylenedioxythiophene):poly (styrene sulfonate) (PEDOT:PSS) channel by a (Cu)porphyrin metal-organic framework (denoted as CuMOF)/BiOBr photogate, which was connected by a salt bridge to prevent any adverse effects on the transistor’s functionality. Upon regulation by different biomolecules in aqueous electrolytes, unique dual-response characteristics of the as-developed OPECT could be produced based on the altered conductive properties of the PEDOT:PSS channel, and the parallel visual adaptation and memory could thus be on-demand generated, highly akin to that in biology [19].
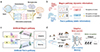 |
Figure 1 Aqueous neuromorphic motion recognition in a real scene. (a) Schematic illustrating the biological signal transduction in human visual system. (b) Parallel Magno pathway processing dynamic information and Parvo pathway processing static information. (c) Artificial AA-mediated Magno pathway and DA-mediated Parvo pathway. (d) Integrated workflow combining AA-mediated transient filtering for motion capture and DA-modulated weight updating for convolutional recognition. |
Specifically, the biosensor exhibited an initial signal enhancement followed by a rapid attenuation response—adaptation—in ascorbic acid (AA) solution, while it demonstrated a monotonic signal increment to a plateau state—memory—in the dopamine (DA) solution. Such dual responses well mimicked those of biology [53,54], rendering it suitable for integrated image filtering in a real scene and recognition of moving objects by artificial neural networks (ANN) (Figure 1d). In the preprocessing stage, the adaptation function can be utilized to filter out stationary objects, capturing and outputting dynamic objects. During the next computational stage, the memory function can be employed within the backpropagation algorithm to update synaptic weights for improved image recognition. This work features the bioinspired parallel emulation of visual adaptation and memory towards biochemically mediated motion recognition in a real scene.
RESULTS
Materials characterization
The morphologies of the gate materials were characterized by scanning electron microscopy (SEM) (Figure S1). X-ray diffraction (XRD) patterns confirmed the preparation of CuMOF, BiOBr, and the CuMOF/BiOBr heterojunction (Figure 2a). The XRD pattern of CuMOF agreed with the previous report [55] (CCDC card no. 675375), while the characteristic peaks of BiOBr demonstrated the tetragonal lattice structure (JCPDS#09-0393) [56]. Besides, after being coupled with CuMOF, the primary crystal facets of BiOBr had not undergone substantial alterations, which was consistent with previous literature [57]. Ultraviolet-visible absorption spectra (Figure 2b) demonstrated the reduction of the Q-band and a red shift in the Soret band of CuMOF, verifying successful coordination between Cu and porphyrin [57]. BiOBr showed a characteristic absorption at approximately 307 nm, aligning with reported data [58]. Notably, the strong light absorption capability of CuMOF effectively extended the optical absorption range of the heterojunction. X-ray photoelectron spectroscopy (XPS) analysis further corroborated the successful synthesis of CuMOF, BiOBr and the heterojunction [58]. Distinct characteristic peaks were identified at 940 eV (Cu), 159 eV (Bi), and 69 eV (Br), in addition to the signals from C, O, and N elements (Figure 2c).
 |
Figure 2 Materials characterization. (a) XRD patterns, (b) UV-vis absorption spectra, and (c) XPS spectra of the CuMOF, BiOBr and heterojunction. The photoelectrochemical responses of the heterojunction to (d) AA (25, 50, 100 mM) and (e) DA (0.5, 1.0, 5.0 mM). (f) The dual-directional signaling behaviors of the OPECT device, with the current change (ΔIDS) corresponding to the postsynaptic current. |
Furthermore, Tauc plots from ultraviolet-visible (UV-vis), Mott-Schottky plots, electrochemical impedance spectroscopy (EIS), Nyquist plots and photoelectrochemistry (PEC) tests were conducted to confirm the band alignment and revealed the formation of a characteristic type-II heterojunction [59] between CuMOF and BiOBr (Figures S2–S6). Subsequently, the photoelectrochemical property of the heterojunction electrode was studied. The electrode was respectively immersed in AA and DA solutions with different concentrations, followed by evaluation of the photo-responsive behaviors under intermittent irradiation (λ=450 nm, density=10.75 mW/cm2) with 10 s illumination cycles. The results showed that the electrode presented increased anodic responses in AA solution of enhanced concentrations (25, 50 and 100 mM), and all of the photocurrents had obvious decay processes (Figure 2d), which were attributed to the back reduction of redox species [60,61] and rapid surface state recombination [62,63], as evidenced by the EIS measurement (Figure S4). By contrast, in the DA solution of enhanced concentrations (0.5, 1.0 and 5.0 mM), the electrode generated enhanced cathode photocurrents but with no decay during the tests (Figure 2e). Further, the electrochemical properties and stability of the PEDOT:PSS channel were tested by the Ag/AgCl gate electrode (Figure S7). Upon assembling the OPECT consisted of the heterojunction-gated PEDOT:PSS channel, analogous bipolar behaviors of the channel currents were observed (Figure 2f). As shown, the presence of AA induced inhibitory (IPSC) with obvious adaptation behaviors, while the presence of DA induced excitatory postsynaptic currents (EPSC) with clear memory. Such adaptation and memory behaviors could be attributed to the unique migration of ions between the photogate and channel in the OPECT (Figures S8 and S9). We finally used these properties in the artificial Magno and Parvo pathways to complete dynamic information filtering and image recognition, respectively. Significantly, different from previous visual adaptation [64] and memory [65] in solid-state devices, these results revealed the co-location of biologically switchable visual adaptation and memory in our aqueous neuromorphic device.
Synaptic plasticity
The synaptic weight in biological synapses was regulated by spike parameters, e.g., intensity, duration, number, and frequency, exhibiting distinct spike-dependent plasticity, including spike-intensity-dependent plasticity (SIDP), spike-number-dependent plasticity (SNDP), spike-width-dependent plasticity (SWDP) and spike-frequency-dependent plasticity (SFDP). These properties of our device were then respectively studied in the presence of AA (50 mM) and DA (1.0 mM) electrolytes.
In the presence of AA, upon increased light intensity from 4.73 to 17.53 mW/cm2, the SIDP displayed an improved ΔIPSC (peak values) from 1.91 to 3.67 μA (Figure 3a). The transient current consistently demonstrated a biphasic trajectory, which initially ascended to a maximum followed by a decay, eventually lower than the baseline. This phenomenon was due to the strong attenuation of the gate photocurrent in the negative direction. Upon pulse-width extension from 1 to 20 s, the SWDP induced different forms of ΔIPSC. The ΔIPSC could remain above the baseline when the pulse-width was short (1 and 2 s), while prolonged pulse-widths (5, 10 and 20 s) led to decays below baseline, as indicated by the blue dots highlighting off-state current (Figure 3b). Notably, the dependence of positive and negative ΔIPSC upon pulse-widths provided a possibility for sorting and filtering the information of different time scales. Upon increasing the frequency from 0.11 to 0.67 Hz, the SFDP demonstrated lower peak values of ΔIPSC, which was ascribed to the improved relaxation in adaptive restoration of the device (Figure 3c). The corresponding ΔIPSC gain was calculated by A10/A1, where the A10 and A1 represented the amplitude of the 10th and 1st IPSC, respectively (Figure S10a). The attenuation of ΔIPSC gain induced by increasing frequency successfully replicated the low-pass filtering characteristics of biological synapses. Besides, as the number of pulses increased from 2 to 10, the SNDP displayed multiple adaptations to achieve lower response states (Figure S10b).
 |
Figure 3 Synaptic plasticity. The AA-mediated (a) SIDP (4.73, 7.95, 10.75, 12.94 and 17.53 mW/cm2 light pulse, 50 mM AA), (b) SWDP (10.75 mW/cm2 light pulse, 50 mM AA), and (c) SFDP (10.75 mW/cm2 light pulse, 50 mM AA). The DA-mediated (d) SIDP (4.73, 7.95, 10.75, 12.94 and 17.53 mW/cm2 light pulse, 1.0 mM DA), (e) SNDP (10.75 mW/cm2 light pulse, 1.0 mM DA), and (f) SFDP (10.75 mW/cm2 light pulse, 1.0 mM DA). |
In the presence of DA, with the increase of light intensity from 4.73 to 17.53 mW/cm2, the SIDP displayed an expected increased ΔEPSC from 67.87 to 91.8 μA (Figure 3d). With the pulse number increasing from 2 to 10, the SNDP exhibited saw-tooth increases in ΔEPSC, with more zigzags, slower declines, and higher peaks accompanied by gradually diminishing gains, which mimicked the biological synaptic strengthening under repeated stimuli (Figure 3e). Notably, the light-dependent updating of the conductance states provided a possibility for weight updating in image recognition. Upon increased frequency from 0.11 to 0.67 Hz, the SFDP demonstrated lower peak values of ΔEPSC, which was ascribed to longer memory decay of the device (Figure 3f). The corresponding ΔEPSC gain (Figure S11a) successfully replicated the high-pass filtering characteristics of biological synapses. Besides, as the pulse-widths increased from 1 to 20 s, the SWDP exhibited increases in ΔEPSC, which could be attributed to the continuous generation of charge carriers (Figure S11b). The changes in currents during the overall processes followed the same trend as the variations in peak values.
Real scene motion recognition
As aforementioned, adaptation is an essential function of biological vision for filtering dynamic information. We have demonstrated that the AA-mediated SWDP could provide such a function in the artificial Magno pathway. As illustrated (Figure 4a), the information within key frames extracted from videos was represented in the pixel grid, where the residence time of dynamic information (td) in the same pixel grid was significantly shorter than that of static information (ts). Besides, depending on the speed of dynamic objects, the residence times within the same pixel grid, denoted as td, was distinct, indicating that filtering dynamic information with varying speeds required distinct time scales. To differentiate the dynamic and static information (Figure 4b), the dynamic information corresponding to the short pulse-width was kept (i.e., the shaded parts of the ΔIPSC regions were utilized), while the static information corresponding to the prolonged pulse-width was discarded (i.e., the positive ΔIPSC regions were abandoned). At the light intensity of 4.73 mW/cm2, as the AA concentration increased, the peak current (Imax) increased from 0.91 to 4.10 μA, while the time-to-peak (tmax) and return-to-zero time (t0) of the currents exhibited corresponding enhancement from 0.6 to 1.2 s and from 1.91 to 11.35 s, respectively. Such a behavior was in principle ideal to match the different td of dynamic objects in the motion recognition. Specifically, the t0 served as the threshold for td, which determined whether the information could be retained. The tmax represented the optimal td at a certain AA concentration. When td=tmax, the ΔIPSC reached the maximum value, which corresponded to the associated grayscale value. The greater the ΔIPSC, the higher contrast between the extracted information and the background for better clarity.
 |
Figure 4 Prerequisite properties for motion detection. (a) Schematic diagram of frame pixel processing and time-scale of dynamic and static information at different moving speeds. ΔIPSC responses at (b) different AA concentrations (25, 50, 100 mM, 7.95 mW/cm2) and at (c) different light intensities (4.73, 7.95, 10.75, 12.94 mW/cm2, 25 mM). (d) tmax and t0 at different AA concentrations (25, 50, 100 mM) and different light intensities (4.73, 7.95, 10.75, 12.94 mW/cm2). (e) Schematic diagram of information filtering at different time scales (light intensity of 4.73 mW/cm2, AA of 25 and 100 mM). (f) LTP and LTD curves generated by respective optical and electrical pulses in 0.5, 1.0, 5.0 mM DA solution. |
In addition to AA concentrations, the effect of light intensity on the tmax and t0 was also studied. For 25 mM AA, the increasing light intensity elevated Imax from 1.91 to 3.67 μA, while tmax showed a slight increase from 0.5 to 0.7 s, while t0 showed a slight decrease from 2.52 to 1.91 s, respectively (Figure 4c). The values of tmax and t0 under varying AA concentrations and light intensities were calculated (Figure 4d and Table S1). As shown, the tmax showed a positive correlation with AA concentration due to the enhanced faradaic reaction, while it showed a negative correlation with light intensity that could be attributed to the faster carrier migration upon stronger light stimulation and thus faster attainment of the Imax. As to t0, the enhanced faradaic reaction similarly led to its positive correlations with both concentration and light intensity. Notably, under high concentrations and high light intensities, the enhancements of the currents were uniform, without crossover occurring in the curves.
Using the above results, different AA concentrations could be deployed to perform motion recognition tasks on different time scales (Figure 4e). When td was less than 1.91 s, information could be retained in cases of 25 and 100 mM AA. When td was at 1.91–11.35 s, the information could be retained in the case of 100 mM AA but be discarded in the case of 25 mM. When td was larger than 11.35 s, the information could not be retained in both cases of 25 and 100 mM AA. Except for the time scale, as a reference value for brightness normalization in a dynamic information filtering algorithm, Imax was positively correlated with recognition ability and information differentiation (Figure S12). In subsequent dynamic information processing, the curve with a higher Imax was selected if the information could be retained by different curves. Due to the aforementioned performance, we divided the current curves into two parts based on the peak values and performed function fittings. These fitted functions were then input into the algorithm, where processing results at different times corresponded to current values at those respective times, enabling us to screen dynamic and static information.
On the other hand, memory is an essential function of biological vision for image recognition. We have demonstrated that the DA-mediated SNDP could provide such a function in the artificial Parvo pathway. After extraction by the AA-mediated pathway, image recognition was achieved through the conductance updates dependent on the pulse numbers. We implemented long-term potentiation (LTP) using 10 optical pulses for conductance state updates, followed by 10 negative electrical pulses for long-term depression (LTD)-mediated state resetting in the presence of different DA concentrations (0.5, 1.0, and 5.0 mM) (Figure 4f). The adjustable synaptic weights demonstrated its memory encoding capability in write-erase operations, and the fitted function of the curve could be used in the weight updating process for image recognition (Figure S13).
Taking advantage of such properties, we explored a unique biochemically tunable artificial vision system for dynamic information filtering in a real scene and subsequent image recognition. As illustrated, the dynamic information filtration involved three key steps: extracting key frames of fixed time interval from videos (e.g., 0.5 s interval) (Figure 5a); categorizing these frames based on dual temporal scales (td and ts); selecting appropriate AA concentration to discard static backgrounds to generate motion trajectories (Figure 5b). To be specific, the 25 mM AA solution was eliminated when td was longer than 2.52 s (25 mM, 12.94 mW/cm2), while the 50 mM solution was also excluded when td was longer than 4.45 s (50 mM, 12.94 mW/cm2) and 100 mM became the final selection. For the ts, the 100 mM AA solution was eliminated when ts was shorter than 11.35 s (100 mM, 4.73 mW/cm2), while the 50 mM solution was also excluded when ts was between 4.24 s (50 mM, 4.73 mW/cm2) to 1.91 s and 25 mM became the final selection. These time nodes were t values measured at the corresponding concentrations and light intensities mentioned above. This time-scale adaptive filtering architecture enabled robust motion filtration across diverse tasks.
 |
Figure 5 OPECT motion recognition in a real scene. (a) Schematic representation of extraction of keyframes from the video. (b) Temporal parameters and selection criteria for optimal AA concentrations in dynamic information filtration. (c) DA-mediated ANN architecture in image recognition. (d) Dynamic information retention profiles processed with varying AA concentrations across different temporal scales (td=0.5–2 s; ts=3–16 s). (e) Recognition accuracy metrics with varying DA concentrations derived from ANN-based analysis of filtration outcomes. |
Based on the aforementioned selection criteria, three videos with diverse time scales (td=0.5, 1, 2 s; ts=3, 6, 16 s) were systematically processed using optimal 25, 50, and 100 mM AA solutions, respectively, yielding dynamic information filtering results as demonstrated (Figure 5c). The outputs exhibited successful AA-mediated dynamic information retention as indicated by the clear moving trajectories against discarded backgrounds (e.g., left-side door), achieving non-overlapping motion tracking without any information loss. Incidentally, if the (td, ts) was processed by the non-optimal AA concentration, it will lead to inferior results (Figure S14). Taking the (td=0.5 s, ts=3 s) as an exemplar, while 50 and 100 mM solutions preserved dynamic information with higher grayscale intensity, their application resulted in partial background retention, causing data redundancy and reduced signal-to-noise ratio.
Following the AA-mediated filtering, DA-mediated information recognition was implemented on the processed images by a three-layer ANN (Figure 5d). As shown, we used a three-layered ANN consisting of input neurons, hidden neurons and output neurons, which were fully connected via synaptic weights (Figure S15). The ANN was trained via backpropagation based on the DA-mediated potentiation and depression. Compared with the randomly distributed conductance before learning, the ∆G value of pixels was substantially increased after learning. Correspondingly, upon optimization of the DA concentrations, the trained output exhibited an optimal accuracy of 93.7% in the case of 5.0 mM DA (Figure 5e). The high recognition rate demonstrated the feasibility of DA-mediated weight updating in ANN.
DISCUSSION
In summary, drawing inspiration from the architecture and functionality of the biological visual system, we designed an OPECT biosensor that emulates the parallel Magno and Parvo pathways found in human vision, characterized by the respective procession of dynamic and static visual information. Specifically, by leveraging the unique photoelectrochemical biosensing in the OPECT, the AA-mediated artificial Magno pathway can mimic the aqueous visual adaptation to capture and output dynamic objects, while the DA-mediated artificial Parvo pathway can mimic the memory function for improved image recognition in ANN. The feasibility of applying the as-prepared biosensor towards real scene motion recognition was verified. Compared with current organic transistor devices in aqueous medium, this biosensor distinguished itself from high-level biomimicry in terms of biologically mediated adaptation and memory. Overall, this work featured aqueous neuromorphic vision biosensors capable of motion recognition in a real scene, which was expected to inspire artificial biosystems that more closely resemble the aqueous processes of human vision.
MATERIALS AND METHODS
Reagents
Phosphate-buffered saline (PBS) was purchased from Jiangsu Kaiji Biotechnology Co., Ltd. PEDOT:PSS dispersion (Clevios PH1000) was obtained from Heraeus Ltd. Cu(NO3)2·3H2O, Bi(NO3)·5H2O, HNO3 and AA were purchased from Shanghai Hutest Laboratory Equipment Co., Ltd. Polyvinyl pyrrolidone (PVP), hexadecyltrimethylammonium bromide (CTAB), trifluoroacetic acid (TFA), DA, N,N-dimethylformamide (DMF), and methyl sulfoxide (DMSO) were purchased from Aladdin Biochemical Technology Co., Ltd. Meso-tetra(4-carboxyphenyl)porphine (TCPP) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. Nafion was purchased from Beijing Bailingwei Technology Co., Ltd.
Fabrication of the gate electrode
CuMOF. The preparation process of CuMOF/BiOBr on indium tin oxide (ITO) was as follows. 3.6 mg Cu(NO3)2·3H2O, 0.7 μL TFA and 10 mg PVP were dissolved in a 12 mL mixture of DMF and ethanol (VD:Ve=3:1) and 4.0 mg TCPP dissolved in 4 mL solution of the same formula was added to the above solution tardily. The mixture was then heated to 80 °C for 24 h after 5 min ultrasonic. After cooling, the CuMOF nanosheets were washed in alcohol three times, centrifuged at 8000 r/min and freeze-dried overnight.
BiOBr. Bi(NO3)3·5H2O (0.13 g) dissolved in 50 mL of HNO3 (2 wt%) was dropwise added into 50 mL of CTAB solution (8×10−3 M), and the mixture was ultrasounded and heated in water bath at 80 °C for 3 h. The precipitate of BiOBr nanosheets was washed three times in water and ethanol, followed by ultrasonic and dried for collection.
CuMOF/BiOBr. The 100 mg BiOBr nanosheets were added to 60 mL of alcohol and the homogenized solution was ultrasonicated for 30 min. Then, 3 mg CuMOF solution was added to the mixture and ultrasound treatment was performed for 15 min. The final mixture was stirred and heated in a water bath at 60 °C for 12 h. The product of CuMOF/BiOBr heterojunction was collected by ultrasound and dried overnight at 60 °C.
Gate electrode. CuMOF/BiOBr powder (2 mg) was ultrasonically dispersed in 1 mL ultra-pure water and 1‰ (wt%) Nafion solution was added. The mixture was dripped onto the plasma-treated ITO electrode sheet, dried and then tested.
Fabrication of the transistor
The transistor fabrication involved sequential deposition of chromium (Cr, 10 nm thickness) and gold (Au, 100 nm thickness) layers via magnetron sputtering (Kurt J. Lesker system) onto pre-cleaned glass substrates using a shadow mask with channel dimensions of 6.0 mm×0.2 mm (L×W). Subsequently, a 0.1 mL aliquot of PEDOT:PSS aqueous dispersion containing 5% DMSO (v/v) was spin-coated (4000 r/min, 30 s) onto the Au/Cr electrode channel to generate a uniform polymeric thin film. Thermal annealing was then performed at 180 °C for 1 h under controlled argon atmosphere within a glovebox, followed by natural cooling to ambient temperature.
Physical characterization
The photoelectric property was investigated by a photoelectrochemical/organic transistor detector (Nandaguang, Nanjing, China) and a Keithley 4200 semiconductor parameter analyzer using AA and DA as the electrolytes. The 405 nm illumination was supplied by commercially available light-emitting diodes. SEM and mapping images were characterized by a Hitachi S4800 microscope. XRD patterns were provided by Bruker D8 Advance equipment. UV-vis absorption patterns were presented by Shimadzu UV-3600. EIS data were measured out by Bio-Logic SP-150.
Data availability
The original data are available from corresponding authors upon reasonable request.
Funding
This work was supported by the National Natural Science Foundation of China (22174063 and 22374066) and the Excellent Research Program of Nanjing University (ZYJH004).
Author contributions
W.W.Z. and Y.H.K. conceived the idea and designed the experiments. Y.H.K. and Z.L. performed the experiments and characterization. C.Y. and Z.L. helped in materials synthesis and data analysis. W.W.Z. and Y.H.K. co-wrote the manuscript and gave approval to the final version. All authors discussed the results and commented on the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
Supplementary file provided by the authors. Access Supplementary Material
References
- Indiveri G, Douglas R. Neuromorphic vision sensors. Science 2000; 288: 1189-1190. [Article] [Google Scholar]
- Choi C, Lee GJ, Chang S, et al. Inspiration from visual ecology for advancing multifunctional robotic vision systems: Bio-inspired electronic eyes and neuromorphic image sensors. Adv Mater 2024; 36: e2412252. [Article] [Google Scholar]
- Fu J, Nie C, Sun F, et al. Bionic visual-audio photodetectors with in-sensor perception and preprocessing. Sci Adv 2024; 10: eadk8199. [Article] [Google Scholar]
- Xu L, Liu J, Guo X, et al. Ultrasensitive dim-light neuromorphic vision sensing via momentum-conserved reconfigurable van der Waals heterostructure. Nat Commun 2024; 15: 9011. [Article] [Google Scholar]
- Sun R, Hou Z, Chen Q, et al. Orientation-selective memory switching in Quasi-1D NbSe3 neuromorphic device for omnibearing motion detection. Adv Mater 2025; 37: e2409017. [Article] [Google Scholar]
- Huang PY, Jiang BY, Chen HJ, et al. Neuro-inspired optical sensor array for high-accuracy static image recognition and dynamic trace extraction. Nat Commun 2023; 14: 6736. [Article] [Google Scholar]
- Zhang Z, Wang S, Liu C, et al. All-in-one two-dimensional retinomorphic hardware device for motion detection and recognition. Nat Nanotechnol 2022; 17: 27-32. [Article] [Google Scholar]
- Chen J, Zhou Z, Kim BJ, et al. Optoelectronic graded neurons for bioinspired in-sensor motion perception. Nat Nanotechnol 2023; 18: 882-888. [Article] [Google Scholar]
- Liao F, Zhou F, Chai Y. Neuromorphic vision sensors: Principle, progress and perspectives. J Semicond 2021; 42: 013105. [Article] [Google Scholar]
- Kudithipudi D, Schuman C, Vineyard CM, et al. Neuromorphic computing at scale. Nature 2025; 637: 801-812. [Article] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: Anatomy, physiology, and perception. Science 1988; 240: 740-749. [Article] [Google Scholar]
- Zhu S, Xie T, Lv Z, et al. Hierarchies in visual pathway: Functions and inspired artificial vision. Adv Mater 2023; 36: 2301986. [Article] [Google Scholar]
- Yau KW, Hardie RC. Phototransduction motifs and variations. Cell 2009; 139: 246-264. [Article] [Google Scholar]
- Gu L, Poddar S, Lin Y, et al. A biomimetic eye with a hemispherical perovskite nanowire array retina. Nature 2020; 581: 278-282. [Article] [Google Scholar]
- Chen K, Hu H, Song I, et al. Organic optoelectronic synapse based on photon-modulated electrochemical doping. Nat Photon 2023; 17: 629-637. [Article] [Google Scholar]
- Yu SY, Hu J, Li Z, et al. Metal-organic framework nanofluidic synapse. J Am Chem Soc 2024; 146: 27022-27029. [Article] [Google Scholar]
- Duan Z, Xu Y, Li Z, et al. Neuromorphic nanofluidic sense digitalization. Angew Chem Int Ed 2025; 64: e202420602. [Article] [Google Scholar]
- Li Z, Chen MH, Wu QQ, et al. A metal-organic framework neuron. Natl Sci Rev 2025; 12: nwaf213. [Article] [Google Scholar]
- Chen Y, Han B, Gobbi M, et al. Responsive molecules for organic neuromorphic devices: Harnessing memory diversification. Adv Mater 2025; 37: 2418281. [Article] [Google Scholar]
- Xu YT, Yu SY, Li Z, et al. A nanofluidic spiking synapse. Proc Natl Acad Sci USA 2024; 121: e2403143121. [Article] [Google Scholar]
- Zhao X, Zou H, Wang M, et al. Conformal neuromorphic bioelectronics for sense digitalization. Adv Mater 2024; 36: 2403444. [Article] [Google Scholar]
- Wu Q, Li Z, Chen M, et al. Reticular photoelectrochemical transistor with biochemical metaplasticity. Adv Mater 2025; : 2504338. [Article] [Google Scholar]
- Huang W, Chen J, Yao Y, et al. Vertical organic electrochemical transistors for complementary circuits. Nature 2023; 613: 496-502. [Article] [CrossRef] [PubMed] [Google Scholar]
- Chen X, Marks A, Paulsen BD, et al. n-Type rigid semiconducting polymers bearing oligo(ethylene glycol) side chains for high-performance organic electrochemical transistors. Angew Chem Int Ed 2020; 60: 9368-9373. [Article] [Google Scholar]
- Xu X, Zhang H, Shao L, et al. An aqueous electrolyte gated artificial synapse with synaptic plasticity selectively mediated by biomolecules. Angew Chem Int Ed 2023; 62: e202302723. [Article] [Google Scholar]
- Yao Y, Pankow RM, Huang W, et al. An organic electrochemical neuron for a neuromorphic perception system. Proc Natl Acad Sci USA 2025; 122: e2414879122. [Article] [Google Scholar]
- Laswick Z, Wu X, Surendran A, et al. Tunable anti-ambipolar vertical bilayer organic electrochemical transistor enable neuromorphic retinal pathway. Nat Commun 2024; 15: 6309. [Article] [Google Scholar]
- Song J, Liu H, Zhao Z, et al. 2D metal-organic frameworks for ultraflexible electrochemical transistors with high transconductance and fast response speeds. Sci Adv 2023; 9: eadd9627. [Article] [Google Scholar]
- Cong S, Chen J, Xie M, et al. Single ambipolar OECT-based inverter with volatility and nonvolatility on demand. Sci Adv 2024; 10: eadq9405. [Article] [Google Scholar]
- Wang S, Chen X, Zhao C, et al. An organic electrochemical transistor for multi-modal sensing, memory and processing. Nat Electron 2023; 6: 281-291. [Article] [Google Scholar]
- Lobosco A, Lubrano C, Rana D, et al. Enzyme-mediated organic neurohybrid synapses. Adv Mater 2024; 36: 2409614. [Article] [Google Scholar]
- Harikesh PC, Yang CY, Wu HY, et al. Ion-tunable antiambipolarity in mixed ion-electron conducting polymers enables biorealistic organic electrochemical neurons. Nat Mater 2023; 22: 242-248. [Article] [Google Scholar]
- Qiu J, Chen P, Wang M, et al. Compact artificial synapse-neuron module with chemically mediated spiking behaviors. ACS Nano 2025; 19: 12298-12307. [Article] [Google Scholar]
- Zhao WW, Xu JJ, Chen HY. Photoelectrochemical bioanalysis: The state of the art. Chem Soc Rev 2015; 44: 729-741. [Article] [Google Scholar]
- Zhao WW, Xu JJ, Chen HY. Photoelectrochemical DNA biosensors. Chem Rev 2014; 114: 7421-7441. [Article] [Google Scholar]
- Ruan Y, Chen F, Xu Y, et al. An integrated photoelectrochemical nanotool for intracellular drug delivery and evaluation of treatment effect. Angew Chem Int Ed 2021; 60: 25762-25765. [Article] [Google Scholar]
- Wang H, Xu Y, Wang B, et al. A photoelectrochemical nanoreactor for single-cell sampling and near zero-background faradaic detection of intracellular microRNA. Angew Chem Int Ed 2022; 61: e202212752. [Article] [Google Scholar]
- Hu J, Lu M, Chen F, et al. Multifunctional hydrogel hybrid-gated organic photoelectrochemical transistor for biosensing. Adv Funct Mater 2022; 32: 2109046. [Article] [Google Scholar]
- Gao G, Chen J, Jing M, et al. Functional metal-organic frameworks for maximizing transconductance of organic photoelectrochemical transistor at zero gate bias and biological interfacing application. Adv Funct Mater 2023; 33: 2300580. [Article] [Google Scholar]
- Hu J, Li Z, Huang Y, et al. Nanocomposite hydrogel enables color-gated organic photoelectrochemical transistor biodetection. Adv Funct Mater 2024; 35: 2412928. [Article] [Google Scholar]
- Wang Z, Shi X, Hu J, et al. PCN-134(Fe)-gated organic photoelectrochemical transistor with unique dual-directional signaling. Adv Funct Mater 2025; 35: 2414037. [Article] [Google Scholar]
- Wang Z, Shi X, Chen F, et al. Ag/AgCl-like photogating of a COF-on-MOF heterojunction in organic photoelectrochemical transistor. Adv Funct Mater 2024; 34: 2404497. [Article] [Google Scholar]
- Wang C, Jiang Y, Li Z, et al. Graphene photoelectrochemical transistor for dual-directional signal-on biosensing. Adv Funct Mater 2025; 35: 2500235. [Article] [Google Scholar]
- Druet V, Ohayon D, Petoukhoff CE, et al. A single n-type semiconducting polymer-based photo-electrochemical transistor. Nat Commun 2023; 14: 5481. [Article] [Google Scholar]
- Corrado F, Bruno U, Prato M, et al. Azobenzene-based optoelectronic transistors for neurohybrid building blocks. Nat Commun 2023; 14: 6760. [Article] [Google Scholar]
- Hu J, Jing M, Huang Y, et al. A photoelectrochemical retinomorphic synapse. Adv Mater 2024; 36: e2405887. [Article] [Google Scholar]
- Huang Y, Li Z, Yuan C, et al. Organic photoelectrochemical multisensory integration. Adv Mater 2025; 37: 2503030. [Article] [Google Scholar]
- Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: An integrated systems perspective. Science 1992; 255: 419-423. [Article] [Google Scholar]
- Sincich LC, Horton JC. Divided by cytochrome oxidase: A map of the projections from V1 to V2 in macaques. Science 2002; 295: 1734-1737. [Article] [Google Scholar]
- Tamietto M, Morrone MC. Visual plasticity: Blindsight bridges anatomy and function in the visual system. Curr Biol 2016; 26: R70-R73. [Article] [Google Scholar]
- Freud E, Plaut DC, Behrmann M. ‘What’ is happening in the dorsal visual pathway. Trends Cogn Sci 2016; 20: 773-784. [Article] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, et al. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn Sci 2013; 17: 26-49. [Article] [Google Scholar]
- Koshland DE Jr., Goldbeter A, Stock JB. Amplification and adaptation in regulatory and sensory systems. Science 1982; 217: 220-225. [Article] [Google Scholar]
- Solomon SG, Peirce JW, Dhruv NT, et al. Profound contrast adaptation early in the visual pathway. Neuron 2004; 42: 155-162. [Article] [Google Scholar]
- Cao X, Huang A, Liang C, et al. Engineering lattice disorder on a photocatalyst: photochromic BiOBr nanosheets enhance activation of aromatic C–H bonds via water oxidation. J Am Chem Soc 2022; 144: 3386-3397. [Article] [Google Scholar]
- Fan C, Lai J, Shao Z, et al. Target-induced photocurrent-polarity-switching PEC sensing platform based on in situ generation of oxygen vacancy-modulated energy band structures. Anal Chem 2023; 95: 15049-15056. [Article] [Google Scholar]
- Zhao M, Wang Y, Ma Q, et al. Ultrathin 2D metal-organic framework nanosheets. Adv Mater 2015; 27: 7372-7378. [Article] [CrossRef] [PubMed] [Google Scholar]
- Zhao Y, Wang J, Pei R. Micron-sized ultrathin metal-organic framework sheet. J Am Chem Soc 2020; 142: 10331-10336. [Article] [Google Scholar]
- Zhang G, Wu H, Chen D, et al. A mini-review on ZnIn2S4-based photocatalysts for energy and environmental application. Green Energy Environ 2022; 7: 176-204. [Article] [Google Scholar]
- Eisenberg D, Ahn HS, Bard AJ. Enhanced photoelectrochemical water oxidation on bismuth vanadate by electrodeposition of amorphous titanium dioxide. J Am Chem Soc 2014; 136: 14011-14014. [Article] [Google Scholar]
- Seo D, Won S, Kim JT, et al. Adopting back reduction current as an additional output signal for achieving photoelectrochemical differentiated detection. Anal Chem 2022; 94: 2063-2071. [Article] [Google Scholar]
- Peter LM. Dynamic aspects of semiconductor photoelectrochemistry. Chem Rev 1990; 90: 753-769. [Article] [Google Scholar]
- Qiu J, Hajibabaei H, Nellist MR, et al. Catalyst deposition on photoanodes: the roles of intrinsic catalytic activity, catalyst electrical conductivity, and semiconductor morphology. ACS Energy Lett 2018; 3: 961-969. [Article] [Google Scholar]
- Li L, Li S, Wang W, et al. Adaptative machine vision with microsecond-level accurate perception beyond human retina. Nat Commun 2024; 15: 6261. [Article] [Google Scholar]
- Zhao T, Yue W, Deng Q, et al. Neuromorphic transistors integrating photo-sensor, optical memory and visual synapses for artificial vision application. Adv Mater 2025; 37: 2419208. [Article] [Google Scholar]
All Figures
 |
Figure 1 Aqueous neuromorphic motion recognition in a real scene. (a) Schematic illustrating the biological signal transduction in human visual system. (b) Parallel Magno pathway processing dynamic information and Parvo pathway processing static information. (c) Artificial AA-mediated Magno pathway and DA-mediated Parvo pathway. (d) Integrated workflow combining AA-mediated transient filtering for motion capture and DA-modulated weight updating for convolutional recognition. |
| In the text | |
 |
Figure 2 Materials characterization. (a) XRD patterns, (b) UV-vis absorption spectra, and (c) XPS spectra of the CuMOF, BiOBr and heterojunction. The photoelectrochemical responses of the heterojunction to (d) AA (25, 50, 100 mM) and (e) DA (0.5, 1.0, 5.0 mM). (f) The dual-directional signaling behaviors of the OPECT device, with the current change (ΔIDS) corresponding to the postsynaptic current. |
| In the text | |
 |
Figure 3 Synaptic plasticity. The AA-mediated (a) SIDP (4.73, 7.95, 10.75, 12.94 and 17.53 mW/cm2 light pulse, 50 mM AA), (b) SWDP (10.75 mW/cm2 light pulse, 50 mM AA), and (c) SFDP (10.75 mW/cm2 light pulse, 50 mM AA). The DA-mediated (d) SIDP (4.73, 7.95, 10.75, 12.94 and 17.53 mW/cm2 light pulse, 1.0 mM DA), (e) SNDP (10.75 mW/cm2 light pulse, 1.0 mM DA), and (f) SFDP (10.75 mW/cm2 light pulse, 1.0 mM DA). |
| In the text | |
 |
Figure 4 Prerequisite properties for motion detection. (a) Schematic diagram of frame pixel processing and time-scale of dynamic and static information at different moving speeds. ΔIPSC responses at (b) different AA concentrations (25, 50, 100 mM, 7.95 mW/cm2) and at (c) different light intensities (4.73, 7.95, 10.75, 12.94 mW/cm2, 25 mM). (d) tmax and t0 at different AA concentrations (25, 50, 100 mM) and different light intensities (4.73, 7.95, 10.75, 12.94 mW/cm2). (e) Schematic diagram of information filtering at different time scales (light intensity of 4.73 mW/cm2, AA of 25 and 100 mM). (f) LTP and LTD curves generated by respective optical and electrical pulses in 0.5, 1.0, 5.0 mM DA solution. |
| In the text | |
 |
Figure 5 OPECT motion recognition in a real scene. (a) Schematic representation of extraction of keyframes from the video. (b) Temporal parameters and selection criteria for optimal AA concentrations in dynamic information filtration. (c) DA-mediated ANN architecture in image recognition. (d) Dynamic information retention profiles processed with varying AA concentrations across different temporal scales (td=0.5–2 s; ts=3–16 s). (e) Recognition accuracy metrics with varying DA concentrations derived from ANN-based analysis of filtration outcomes. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

