| Issue |
Natl Sci Open
Volume 4, Number 1, 2025
Special Topic: Nuclear Environment Advances
|
|
|---|---|---|
| Article Number | 20240024 | |
| Number of page(s) | 37 | |
| Section | Earth and Environmental Sciences | |
| DOI | https://doi.org/10.1360/nso/20240024 | |
| Published online | 19 September 2024 | |
REVIEW
Current understanding and challenges of photocatalytic reduction of U(VI) using polymeric photocatalysts
1
Jiangxi Province Key Laboratory of Functional Organic Polymers, East China University of Technology, Nanchang 330013, China
2
National Key Laboratory of Prospecting, Mining and Remote Sense Detecting on Uranium Resources, East China University of Technology, Nanchang 330013, China
3
School of Applied Engineering, Gandong University, Fuzhou 344000, China
* Corresponding authors (emails: This email address is being protected from spambots. You need JavaScript enabled to view it.
(Yunhai Liu); This email address is being protected from spambots. You need JavaScript enabled to view it.
(Zhibin Zhang))
Received:
31
May
2024
Revised:
24
July
2024
Accepted:
8
August
2024
Abstract
Uranium, vital for nuclear fuel production, holds immense potential in both the nuclear and environmental sectors. Rising demand for uranium is accompanied by dwindling ore reserves, thus impeding the advancement of sustainable nuclear energy development. Therefore, it is imperative to extract uranium from seawater and radioactive wastewater to ensure the effective utilization of uranium resources and the conservation of the natural environment. Non-homogeneous photocatalysis is recognized for its safe operation, eco-friendly characteristics, and energy-saving functions, which have received considerable attention recently. Given their molecularly adjustable structure, polymer semiconductors, given their molecularly adjustable structure, have demonstrated superior catalytic performance. A marked improvement in photochemical conversion efficiency has been observed with the introduction of advanced synthesis methods. This review aims to elucidate the principle of photocatalytic uranium remediation, critically examining the structural design and uranium removal efficacy of diverse polymer semiconductors, with emphasis on factors such as light absorption, charge separation, and stability. We then delve into the mechanisms of uranium removal by these materials under different environmental conditions, emphasizing their impact on carrier separation and transport, and the reduction of uranium products. Finally, the challenges and prospects of polymer semiconductor photocatalysis in the removal of U(VI) are discussed.
Key words: uranium / polymeric photocatalysts / photocatalytic / charge separation
© The Author(s) 2024. Published by Science Press and EDP Sciences.
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Background of the photoreduction of U(VI)
Nuclear power is the second largest source of clean power after hydropower, which has made a significant contribution to global emissions reduction to date. In the future, it can help people reduce the dependence of power structures on fossil fuels, reduce carbon emissions, and achieve the goal of zero net emissions [1]. However, the vigorous development of nuclear energy is limited by two bottlenecks. On the one hand, Uranium, as one of the crucial fuels for the advancement of the nuclear industry, is relatively scarce in China, with the nation importing about 80% of its demand, surpassing various international warning thresholds [2]. Although uranium is primarily obtained from ore and subsequently refined for the nuclear energy development, mining activities can generate radioactive wastewater, which poses a significant environmental and health risk. Therefore, the development of efficient uranium wastewater treatment technology is imperative. Alternatively, the uranium reserve in seawater is 1000 times that of the terrestrial reservations, which is more than 4.5 billion tons, as a potential sustainable uranium supply warehouse in the future. Unfortunately, the process of separating uranium from seawater is very complicated, mainly because the existing technology not only separates uranium but also introduces impurities such as various soluble organics (including citric acid, oxalic acid, ethylene diamine tetra acetic acid, tannic acid, sulfamate, etc.) and coexisting ions (such as vanadium, cobalt) present in seawater. Therefore, seawater uranium extraction (UES) is also recognized as a groundbreaking chemical separation technology [3]. On the other hand, the uranium content in drinking water is at μg/L level, and that in natural surface water is at mg/L level, during the front end or the back end of the nuclear fuel cycle [4]. As a typical case, the discharge of nuclear wastewater from Fukushima and the treatment of uranium mine wastewater have attracted much attention. Therefore, uranium pollution must be eliminated before radioactive waste is finally disposed of in geological repository.
Recent advances have led to various techniques for separating and enriching uranium, such as adsorption (electro-adsorption [5] and chemical adsorption [6]), membrane separation [7], and reduction methods (biological reduction, chemical reduction, and photoreduction). Among them, photocatalytic reduction has gained popularity among researchers due to its ability to efficiently convert the free state of U(VI) to the solid state of U(IV) and achieve the purpose of separation and enrichment, increasingly favored by researchers [8–10]. Among them, photocatalytic reduction has gained popularity among researchers due to its ability to efficiently convert the free state of U(VI) to the solid state of U(IV) and achieve the purpose of separation and enrichment, which is increasingly favored by researchers [11]. Compared to electrocatalytic and chemical reduction, the advantages of photocatalytic U(VI) reduction revolve around two essential points. First, photocatalytic reduction benefits from a wide range of energy sources. Photocatalytic reduction transforms solar energy into electricity, demonstrating green sustainability, low cost, and user-friendliness. Second, photocatalytic uranium reduction typically accompanies oxidation of organic matter in wastewater. Specifically, electrons and holes generated from semiconductor catalysts photochemically induce oxidation and reduction. In the process of photocatalytic uranium reduction in wastewater, organic waste in wastewater is oxidized. Therefore, photocatalytic semiconductor reduction not only achieves photochemical uranium reduction, but also achieves organic matter oxidation in wastewater. The photocatalytic reduction of U(VI) on TiO2 by Amadelli and his co-worker opened up new possibilities for exploring photocatalysts for charge transport applications [12]. Subsequently, research related to U(VI) photoreduction has grown rapidly every year.
Mechanism of the photoreduction of U(VI)
Photocatalytic reactions rely heavily on the properties of certain semiconductors are especially suitable due to their redox potential falling between E(CB) and E(VB). These semiconductors are particularly effective in assisting the photocatalytic degradation of substances with redox potentials below U(VI) [13]. While the band gap is certainly a key factor, the photo-absorption range is equally crucial in determining their efficiency. Under sunlight, most of our solar radiation falls within the visible spectrum, comprising approximately 50% of the total, while the ultraviolet portion accounts for only 7%. Unfortunately, many photocatalysts’ absorption ranges fall within this less efficient ultraviolet region. Therefore, it is crucial to develop catalysts that can effectively utilize visible light. Polymer photocatalysts are gaining popularity for their non-toxic nature, high surface area, ease of synthesis, and adjustable bandgap and light absorption range, making them excellent candidates for photocatalytic degradation of uranium.
Figure 1 illustrates a potential photocatalytic reduction pathway of U(VI) on polymer semiconductor photocatalysts under solar irradiation. Upon exposure to light at λ ≥400 nm, a significant number of e−-h+ pairs are formed on the catalyst’s surface. This electric field effectively separates them. Electrons swiftly transfer from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO)through a quick charge transfer path, while holes remain in the HOMO. Active electrons promptly convert the adsorbed UO22+ to UO2. In this step, photogenerated electrons join with oxygen to generate reduced ·O2− radicals and H2O2, which then transform the adsorbed UO22+ into the active species (UO2)O2·2H2O. A built-in electric field optimally separates the photogenerated carrier pairs. Electrons rapidly migrate from HOMO to LUMO through an efficient charge migration channel, leaving an equal number of holes in the HOMO. Finally, some electrons directly reduce UO2+ adhesion to the catalyst surface to UO2. Meanwhile, a significant portion of the electrons interact strongly with oxygen to produce ·O2− and H2O2, further reducing the adsorbed UO22+ to (UO2)O2·2H2O.
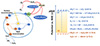 |
Figure 1 Possible mechanism for photocatalytic reduction of uranium by polymers. |
To better understand and utilize the mechanisms of uranium photoreduction, it is crucial to incorporate certain key factors into the design of our polymer photocatalyst for U(VI) photoreduction. These key principles revolve around understanding and controlling of the energy band structure, band gap, carrier transfer effect, and the creation of photoactive U(VI) adsorption sites [14]. The optical absorption threshold (λg) is inversely proportional to the band gap energy (Eg) (λg = 1240/Eg). Therefore, Eg directly influences the wavelength range of optical absorption. For a unique energy band structure, photocatalysts for uranium reduction require a higher conduction band than the reduction potential of uranium (+0.41 V) to facilitate electron transfer [15]. In general, the reduction potential of uranium is highly dependent on the uranium species, which are UO22+, UO2CO3, and UO2(CO3)34− at pH < 4, 5 < pH < 7, and pH > 7, respectively. By converting the vacuum energy level and the chemical energy level, we derived equations indicating photocatalytic reduction of uranium (vs NHE) as illustrated in Figure 1b.
The precise mechanisms and adsorption sites involved in the photocatalytic reduction of U(VI) are of great significance in the design of effective photocatalysts for this process. These sites offer a unique advantage compared to other photocatalytic reactions. These sites promote intimate contact between U(VI) and the catalyst surface, which is essential for photocatalytic uranium reduction. When U(VI) adsorbs on these sites, it becomes an electron capture site, facilitating electron acquisition and reduction. Therefore, the key to promoting photocatalytic uranium reduction of photocatalysts lies in regulating the energy band structure, improving charge separation efficiency, and creating efficient U(VI) adsorption sites.
Advances in the study of polymeric photocatalysts
In this context, more photocatalysts are being used for uranium reduction, with polymeric photocatalysts standing out due to their metal-free nature. These polymeric semiconductors have a wider range of configurations than other photocatalysts (complexes, metals, and inorganic semiconductors). They include various monomer compositions, flexible chains, and spatial variations. These photocatalysts primarily comprise conjugated polymers (CPs) [16], graphitic carbon nitride polymer (g-C3N4) [17] conjugated microporous polymers (CMPs) [18], covalent organic frameworks (COFs) [19], hydrogen-bonded organic framework (HOF) [20], and metal-organic framework (MOFs) [21]. Notably, CMPs, a microporous polymer with high specific surface area and conjugation, are often composed of two or more monomer species. Given their unique properties and potential uses, these microporous materials have attracted significant interest. However, it is important to note that most CMPs lack long-range order, resulting in an amorphous structure. While this structure offers many reactive sites and improves charge separation, it makes it challenging to assess its specific impact on photocatalytic performance. Recently, advanced polymer frameworks such as CTFs, COFs, HOFs, and MOFs have been introduced to enhance these amorphous polymeric photocatalysts. These frameworks boast remarkable crystalline and porous structures, leading to intricate catalytic behaviors [22].
This review examines the current understanding of the key factors that govern the reactivity of a material response towards U(VI), building on recent research. This review will focus on bond formation, functional group manipulation, development of synergistic mechanism, electron transport mechanisms, as well as highlight the critical issues and breakthroughs in wastewater remediation and seawater uranium extraction using polymeric photocatalysts. Additionally, this review will also discuss the challenges and perspectives.
IMPORTANT PROPERTIES OF POLYMERIC PHOTOCATALYSTS REQUIRED FOR URANIUM REDUCTION
Managing charge carrier behaviors is key to enhancing material performance, covering generation, separation, migration, and surface reaction. Polymers offer an expansive platform for designing photocatalytic systems that maximize the use of photo-induced carriers due to their large surface area. Various polymer-based methods have been engineered to intentionally manage carrier behaviors. We will categorize these methods for regulating carrier activities neatly, but typically catalysts are crafted to boost various stages of the photocatalytic process, which we will organize into separate sections for in-depth discussion.
Adjustable optical band gap and high efficiency in light absorption
The first step in the photocatalytic process is to absorb light and produce charge carriers. It determines the total number of charge carriers produced by photocatalysis and the maximum utilization efficiency of light. In general, high light intensities improve performance due to the increased a large number of photons. However, photocatalysts could only absorb light whose energy was equal to or greater than its band gap. As a result, under sunlight, widening the range of light absorption and increasing the capacity of absorbing light is a simple method for improving the removal rate of photoreduction U(VI). In this part, we discussed various ways to enhance the photocatalysts capacity to remove U(VI).
The statistical copolymerization of aromatic monomers proven to be an effective method for increasing the catalytic performance of polymeric by enhancing their properties, such as adjustable optical band gap, porosity, and the charge separation. For example, Cooper and coworkers generated fifteen CMPs by varying the feed ratio of the four comonomers [23]. The bandgap of CMP increased from 2.95 to 2.33 eV as the pyrene concentration of the polymer increased.
The dynamic arrangement of electron-rich donor (D) and acceptor (A) moieties in the primary chain may result in intramolecular charge transfer interactions, reducing band gap and altering molecular energy states. Benzothiadiazole (BT), a potent acceptor moiety commonly employed in D-A copolymer synthesis, was notably affected upon substitution onto the central phenyl group, which have been proven by Wang et al. [24]. Another study employed perylene as a donor and tailored acceptors from 9H-fluoren-9-one to dibenzo[b,d]thiophene 5,5-dioxide to generate two original CMPs, was displayed in Figure 2a. Therefore, ECUT-SO with the strongest acceptor exhibits quite high photocatalytic activity for reducing U(VI), achieving 97.8% reduction efficiency within 60 min, exceeding most photocatalysis materials for this application [25]. The doping of foreign elements to the polymer photocatalyst can be incorporated into the crystal lattice of the catalyst, resulting in a narrow band gap which increases the light-absorbing ability of the synthesized photocatalysts [26]. For instance, the absorption edge of poly(heptazine imide) had shifted from 450 nm to 600 nm after K+ and cyano group co-decorated (KCN-PHI), which achieves a high removal rate of U(VI) with a reaction rate of 0.89 min−1 and is 47 times greater than that of primitive carbon nitride (Figure 2b) [27]. Significantly, heteroatom dopants are usually accompanied by the fracture and formation of chemical bonds, so it is difficult to avoid the introduction of vacancies.
 |
Figure 2 (a) Manufacturing process, UV-Vis DRS spectra and bandgaps of conjugated microporous polymers [25]. (b) Estimated band structures of PCN and KCN-PHI [27]. (c) Estimated band structures of CCN and D-CCN [28]. (d) Schematic illustration and DRS spectra of the TTA-UC ternary nanohybrid preparation [31]. (e) Estimated band structures of Co3O4@TiO2@CdS@Au [32]. (f) Calculated spatial distributions of LUMO and HOMO in the Tp-TMT, Hb-TMT and Tb-TMT [35]. |
The mechanism of enhancing photocatalytic light absorption by introducing defects differs from that of heteroatom doping. The introduction of defects into the g-C3N4 backbone can alter the band structure by forming mid-gap states, which extend the absorption of visible light and also serve as trapping sites for charge carriers, restricting the recombination of photogenerated carriers. Wang et al. achieved nearly 8 times increase in HER compared to the pristine polymer by introducing defects into g-C3N4 through NaBH4 reduction of a crystalline pristine polymer under an inert atmosphere (Figure 2c) [28].
Photocatalysis can benefit from up-conversion materials that could shorten the wavelength of incident light, enabling semiconductors with a large band gap to be excited by a short wavelength converged light [29]. For instance, Kim et al. [30] developed a ternary nanohybrid catalyst that utilized two types of chromophores as sensitizers and acceptors to convert red light into green light through up-conversion. Moreover, carbon dots (CDs) with fluorescence capability can transform visible and near-infrared light (800 nm wavelength) to the absorption edge of carbon nitride at around 450 nm (Figure 2d) [31]. The localized surface plasmon resonance (LSPR) effect involves the coherent oscillation of free electrons in the nanoparticles of plasmonic metals in response to an external oscillating electric field, like sunlight irradiation for photocatalysis. This effect is a practical way to utilize low-energy photons efficiently. Metal nanoparticles like gold (Au) (Figure 2e) [32], silver (Ag) [33], and copper (Cu) can generate energetic hot electrons via the LSPR effect under visible light exposure, surpassing the interface Schottky barrier energy and enhancing the light absorption of semiconductor in the visible and near-infrared regions. Notably, the plasmonic absorption properties depend on the intrinsic characteristics and morphological aspects of the plasmonic materials.
Maximizing the photocatalyst’s light absorption can be done optimally through developing narrow-band gap photocatalysts. Currently, the field of metal-free organic photocatalysts is gaining momentum due to concerns about the costs and environmental impact of metal catalysts. For instance, modifying imine COFs post-synthesis yielded the TFPPy–DP, a narrow-bandgap metal-free catalyst. It exhibited excellent light absorption performance across a broad spectrum [34]. Additionally, the introduction of triazine units and hydroxyl groups in COFs narrowed the optical band gap, leading to improved photocatalytic removal of U(VI). The superior sorption capabilities are mainly due to selective sorption, photoreduction, and chemical reduction of U(VI) into insoluble U(IV) (Figure 2f) [35]. By integrating morphological design with cocatalyst decoration, these light-sensitive materials can demonstrate remarkable activity, akin to the SnS2COF Z-scheme van der Waals heterojunction structure used for radioactive contamination reduction in sewage [36].
In addition to the strategies mentioned above, optimizing the use of available photons is another effective method. This involves fine-tuning the light path and duration within the photocatalyst to maximize the limited photon. For instance, the O-doped graphitic carbon nitride nanotubes showed 2.1 times increase in light absorption rate within the 450–800 nm wavelength range, attributed to enhanced light reflection within the tube channels, extending the light path distance and duration [37]. Hollow structured photocatalysts, compared to flat ones, achieve better light scattering, thus boosting photocatalytic performance.
Separation of photogenerated carriers
The role of free carriers in photo-redox catalysis is vital, being charge transfer. The second, crucial step in photocatalysis is charge separation and migration. This step requires charges to move to the surface, where they can interact with reactants in U(VI) photoreduction. However, random charge carrier separation often increases recombination under light irradiation. Hence, effective charge carrier separation has become a hot research topic recently.
Polymers to impede the recombination of photogenerated electron-hole pairs are a promising strategy. Research by Su and colleagues showed that the position of CN groups and carbazole substitutions on the central phenyl ring can affect light absorption and exciton dissociation properties of the polymers [38]. Sun et al. also found that the photocurrent density of TpPa-COF(CH3)2 was notably higher than TpPa-COF-H and TpPa-COF-NO2, suggesting that enhancing electron-donating characteristics of substituents can improve charge separation in COFs [39]. Additionally, organic small molecules can be incorporated into g-C3N4 through copolymerization to adjust its p-electronic system and band structure (Figure 3a). Creating polymer heterojunctions (PHJs) is an efficient way to modify the band structure and charge transport of g-C3N4. Shen et al. successfully developed three PHJs, resulting in enhanced optical performance [40].
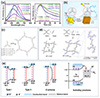 |
Figure 3 (a) The PL and TR-PL of the six CN-modified N-CMPs [40]. Design and synthesis of the (b) PCTF-1 [44], (c) Nx–COFs [45], (d) sp2c-COF and sp2c-COFERDN [47]. (e) Schematic illustration of four types of heterojunctions. |
Heteroatom doping is another effective method to enhance the photocatalytic activity of polymers. The van der Waals radius of a fluorine atom is similar to that of a hydrogen atom (1.2 Å) [41,42]. Zhu and collaborators studied the effect of fluorine substitution on the photocatalytic activities of linear CPs containing BT and conjugated porous polymers [43], resulting in a more negative LUMO level. Similarly, the photocatalytic performance of CTFs could be improved through phosphorus doping, leading to a narrower band gap and more driving force for charge separation (Figure 3b) [44].
Constructing electron donor (D) and acceptor (A) monomer structures in polymer photocatalysts is an effective approach to facilitate carrier migration and enhance photocatalytic performance. The high crystallinity in COFs enhances charge separation, presenting promising catalytic potential. When creating COFs, adjusting the dihedral angles between the central aryl ring and peripheral phenyl rings via hydrogen substitution for nitrogen atoms (as seen in Figure 3c) results in diverse planarity, influencing the crystallinity and porosity of the final product, as evident from their sharper PXRD peaks, which aids in charge migration [45]. Furthermore, the photocatalytic efficiency of COFs can be affected by the linker bond length between comonomers. Thomas and colleagues found that the TP-EDDA displayed notably higher activity than the TP-BDDA, likely due to the increased generation of charge carriers in TP-EDDA [46]. It is believed that fully π-conjugated COFs, compared to those with imine, hydrazone, or azine linkers, could facilitate more efficient carrier separation (Figure 3d) [47].
In addition to the strategies mentioned above, constructing heterojunctions is a highly effective method for spatially separating photogenerated carriers. This process allows electrons excited in one catalyst to transfer to another, leading to longer carrier lifetimes. Specifically, we will explore four types of heterojunctions for U(VI) reduction (Figure 3e): type-I heterojunction, type-II heterojunction, direct Z-scheme heterojunction, and Schottky heterojunction.
(1) Type-I. Electrons and holes in semiconductor-A transfer and gather at the conduction band (E(CB)) and valence band (E(VB)) of semiconductor-B, respectively. Unfortunately, this overlap impedes carrier pair separation. To counteract this, researchers often incorporate sacrificial agents to eliminate excess holes, thus aiding the reduction of U(VI) on materials with lower reduction potential [48].
(2) The Type-II heterojunction provides an intersection of band structures, enabling electron flow to one material and hole flow to another, promoting efficient charge separation at the interface. Constructing these heterostructures using semiconductors and metal-free two-dimensional materials, like g-C3N4, g-C3N5, graphene, and graphene oxide, is a promising strategy [49]. For example, Liu and colleagues synthesized a Type-II heterojunction comprising 2D thin hexagonal SnS2 nanosheets and 2D g-C3N4 nanosheets, demonstrating a rate constant 3.08 times faster than pristine g-CNNs [50]. Additionally, various combinations like C3N5/GO, CdS/g-C3N4, MoS2/g-C3N4, ZnS/g-C3N4, g-C3N4/WS2, COF/BiOBr, and MoS2/P-g-C3N4 have been utilized for the photocatalytic reduction of U(VI), demonstrating improved performance [51–57].
(3) The direct Z-scheme heterojunction photocatalyst offers a more favorable electron migration mechanism due to the electrostatic attraction between photo-induced carriers. Liu and colleagues [36] studied the impact of heterojunction coverage on charge separation efficiency and U(VI) reduction rate in a SnS2-COF van der Waals heterojunction. The spatially separated structure and Z-scheme electron migration pathway in this type of heterojunction enhance charge separation. Similarly, other heterojunctions like g-C3N4/LaFeO3, g-C3N4/Ti3C2, and MoS2/g-C3N4 have been reported to exhibit a Z-scheme electron transfer route, resulting in efficient U(VI) removal [58–60].
(4) Schottky junctions are formed by a metal and a semiconductor, creating a barrier that prevents photogenerated carriers from flowing back from the metal to the semiconductor. In a Schottky junction, the metal acts as a repository for photogenerated carriers. For instance, in the case of MoSx/RGO hybrid nanosheets, a high removal rate of 91.6% was achieved, with nearly 83.4% of U(IV) species being reduced. This high efficiency was attributed to the formation of a metal-semiconductor (M-S) interface, enhancing the spatial separation of photogenerated carriers [61].
Surface kinetics
Enhancing the surface of polymers via strategies like doping and loading can result in intrinsic electric fields at the interface, promoting charge carrier separation, reducing recombination, and boosting reaction sites. Incorporating functional groups (–NH2, –CN, and –C=N) is an advanced approach to boosting photocatalytic efficiency. This chemical alteration can increase carrier pair separation rates, thus improving charge transfer efficiency during U(VI) photoreduction. Plus, these functional groups also serve as U(VI) binding sites, facilitating electron transfer [62]. For example, Gao and colleagues [63] crafted porous g-C3N4 photocatalysts functionalized with heteroatoms and cyanide (–CN) groups. The cyanide addition broadens the photosensitivity range and acts as a strong electron absorption group, enhancing carrier separation. Consequently, the U(VI) photoreduction activity increased by 6.09 times. Similarly, Li et al. [64] synthesized carboxylated carbon nitride for U(VI) photoreduction in a carbonate system, showcasing the efficacy of surface modification in enhancing photocatalytic performance for U(VI) reduction. The low potential of E(CB) and narrow band gap of CCN facilitate the collection of more photons in the conduction band. The U(VI) removal efficiency involves a step enriched on the catalyst surface. Feng et al. have demonstrated that U(VI) adsorption is crucial in the photoreduction process [65]. Given the benefits of adsorption and the properties of uranium, combining adsorption and photocatalysis appears to be a more efficient method. Li, a member of our team, successfully employed PMo12/UiO-66 to enrich U(VI) on the material surface. Under light irradiation, photo-generated electrons swiftly migrate from UiO-66 to PMo12, reducing the pre-enriched U(VI) to U(IV) [66].
Excellent stabilities
Photocatalysts face severe conditions, including nuclear wastewater, uranium mining, and natural water bodies. Interestingly, many polymers are comprised of light elements (C, H, O, N) and can maintain their structure even at 400 °C under inert gas protection [67,68]. Strongly bonded organic polymer photocatalysts have demonstrated resilience in solution. For example, triazine-linked COFs formed through cyclization reactions exhibit exceptional photocatalytic performance and stability [69,70]. The CMPs developed by Liang et al. have high coupling properties and retain good photocatalytic properties after multiple uses [71,72]. Moreover, water movement caused by wind and stirring should be taken into account. Xu et al. crafted a polyacrylonitrile fiber-based photocatalyst with robust mechanical properties [73]. The incorporation of benzoxazole-linked COFs into 3D porous polymer scaffolds (polydimethylsiloxane) has been reported, resulting in polymer sponges that effectively resist seawater erosion and possess good antibacterial properties [74]. In wastewater, diverse organisms can encrust the photocatalyst, thus affecting its performance, selectivity, and lifespan. Hence, photocatalysts ought to resist biological contamination for practical wastewater use [75]. A COF hydrogel with a three-dimensional porous structure, incorporating an electron donor 2,3,6,7-tetra(4-formylphenyl)tetrathiafulvalene, has been introduced. This material can generate biotoxic ROS, rendering it highly antibacterial [76].
The real uranium-containing wastewater possesses elevated levels of impurity ions like Cl− at up to 40 g/L, alongside high concentrations of CO32−, Ca2+, and Mg2+. Such a high chloride level hampers the efficiency of ion exchange in wastewater treatment. Additionally, carbonate ion complexes can hinder U(VI) removal through adsorption techniques [77]. Traditional resin adsorption may present challenges due to economic constraints, salt impurities, and extraction complexities. However, our team’s CdS/UiO-66-NH2 demonstrated an 85.62% removal efficiency in actual uranium-bearing mine wastewater, which is still quite useful [78]. We have also developed a novel hollow nanosphere MnOx@TiO2@CdS@Au (MTCA), which achieved a high U(VI) removal rate of 80.02% even after 60 minutes of simulated sunlight exposure in a high salinity system [79].
There is no doubt that the excellent structural stability of organic polymers provides the prerequisite for their practical application in photocatalytic reactions.
Others
The nanopore density and large surface area of organic polymers offer excellent spots for U(VI) adsorption and reduction during photocatalysis. Larger pores enhance solution permeability, facilitating substance transport. The studied surface area and pore structure of organic polymers are linked to their crystallinity and pore-packing mode, allowing for post-synthesis tuning [80]. The IMDEA-COF-1 crystal structure revealed that the AA stacking pattern equates to a large pore size, while the AB stacking pattern signifies a small pore size, highlighting the impact of the stacking pattern on pore size [81,82]. Miao et al. [83] crafted highly crystalline COF-1 with a large surface area of 2143 m2·g−1, while the corresponding amorphous COP-1 had a surface area of merely 13.8 m2·g−1. Wang et al. [84] investigated the removal of uranium using mesoporous g-C3N4 with adjustable proportions. Mesoporous g-C3N4 exhibited higher efficiency in separating e−-h+ pairs and higher light absorption efficiency compared to the original g-C3N4 [63].
In addition, adding sacrificial agents is a common method to enhance the performance of photocatalysts for reducing U(VI), but the undoubtedly increases the cost of treatment, and some sacrificial agents may also cause secondary pollution to water bodies. Therefore, given the practical application of photocatalysts, it is important to design a photocatalytic system without sacrificial agents [79]. The documented CdS/UiO-66-NH2 can be applied to treat uranium-containing wastewater without sacrificial agents [63,78].
POLYMERIC PHOTOCATALYSTS FOR REDUCING U(VI)
Graphitic carbon nitride (g-C3N4)
Emerging as an impressive n-type semiconductor in non-metallic polymers, g-C3N4 possesses a range of advantageous properties, including unique electrical, optical, and physicochemical attributes, thereby rendering g-C3N4-based materials an attractive multifunctional nanoplatform for various electronic, catalytic, and energy applications [85,86]. g-C3N4 possesses a planar two-dimensional layered structure akin to graphene, wherein triazine rings and 3-s-triazine rings serve as the fundamental structural units that extend endlessly within the plane, forming a network-like structure [17]. The interlayer bonding in g-C3N4 is controlled by van der Waals forces, endowing it with excellent thermal and chemical stability [87].
In 2016, Lu et al. [88] first utilized g-C3N4 for photoreduction of U(VI), which aroused widespread interest in the following years. Compared to TiO2, g-C3N4 has an adjustable band gap (~2.7 eV) and can therefore respond in the visible light range. Furthermore, E(VB) and E(CB) of g-C3N4 are roughly 1.6 eV and −1.1 eV, enabling the photoreduction of U(VI) [89]. However, the inherent low specific surface area and high exciton dissociation energy of polymer semiconductors seriously hinder its application [90]. In response to this situation, various modification strategies have been proposed (Figure 4a, Table 1). As a mature modification strategy, the doping method can effectively optimize the band gap of g-C3N4. In subsequent studies, Lu et al. [91,92] confirmed the universality and effectiveness of this method by doping g-C3N4 with S and P elements, respectively. Taking S-g-C3N4 as an example [93], the doping of S atoms replaces the positions of N atoms in g-C3N4, greatly decreasing the energy gap between them (Figure 4b). Meanwhile, the substitution of S atoms reduces the binding energy of the material to uranyl ions, which in turn enhances the adsorption of U(VI) onto the surface of g-C3N4.
Summary of photocatalytic U(VI) based on g-C3N4 materials
 |
Figure 4 (a) Modification strategy of g-C3N4. (b) Molecular orbital energies and structures of the g-C3N4 and S-g-C3N4 as well as the coordination complexes with U(VI) ion [91]. (c) Schematic diagram of the mechanism of g-C3N4/TiO2 photocatalytic reduction of U(VI) and oxidation of As(III). (d) Photoreduction of U(VI) and the oxidation of As(III) by g-C3N4/TiO2 [94]. (e) Schematic diagram of g-C3N4-CN agar aerogel seawater uranium extraction device. (f) Performance of g-C3N4-CN agar aerogel for uranium extraction from seawater [97]. (g) Mesoporous g-C3N4 photocatalytic U(VI) extraction from seawater. (h) Extractability of mesoporous g-C3N4 for uranium in deionized water and seawater. (i) The U LIII-edge XANES spectra of U(VI)-loaded mesoporous g-C3N4 after irradiation [99]. |
In contrast to the doping method, the construction of heterojunctions primarily aims to address the issue of rapid charge recombination in g-C3N4. Jiang et al. [94] synthesized g-C3N4/TiO2 composite catalysts via a simple calcination method. Under simulated light sources, it was found that 40-CNT exhibited the strongest photo-reduction and photo-oxidation performance. Notably, heterojunction materials exhibit a distinct catalytic mechanism compared to single component materials, where electrons and holes generated under light excitation aggregate at the E(CB) of TiO2 and the E(VB) of g-C3N4, simultaneously reducing U(VI) and oxidizing As(III) (Figure 4c). It has become a consensus that photocatalytic degradation of U(VI) requires an anaerobic environment (Figure 4d). To overcome the oxidation of U(IV) under environmental conditions and reduce the operational difficulty, He et al. [95] prepared WOx/g-C3N4 heterojunction photocatalysts with atom vacancies in recent studies. Research has shown that the introduction of oxygen vacancies regulates the overall band gap structure, enhancing the adsorption and activation of O2. At the oxidation end, benzyl alcohol is fully oxidized to high-value benzaldehyde, while at the reduction end, the photoreduction process avoids the interference of O2. The conversion of uranyl and benzyl alcohol by WOx/g-C3N4 in air atmosphere is more than 98%. In addition, the successful construction of SnS2/g-C3N4 [50], g-C3N4/Ti3C2 [59], ZnS/g-C3N4 [96], etc. demonstrates the effectiveness of heterojunction strategies.
As is well known, g-C3N4 lacks sufficient adsorption sites for U(VI), and the arbitrary introduction of functional groups may lead to low efficiency caused by the inconsistency between adsorption sites and catalytic sites. Hu et al. [97] successfully solved the above problems by preparing cyanide functionalized carbon nitride through a high-temperature polymerization method. Cyanide, as a common electron withdrawing group, can attract a certain range of photo-generated electrons to enhance its photoelectric performance, as well as serve as a complexing group for U(VI) (Figure 4e and f). Therefore, the prepared g-C3N4-CN exhibits excellent photoreduction performance of U(VI), with a maximum uranium adsorption capacity of staggering 2644.3 mg·g−1. It is gratifying that g-C3N4-CN exhibits an exceptional ability to extract uranium from seawater, and its low preparation cost and efficient catalytic performance make the industrial application possible. Coincidentally, Li et al. [98] prepared carboxyl-modified g-C3N4 catalysts (CCN), where surface carboxyl groups altered the chemical form of adsorbed U(VI), making it more easily reduced. The carboxyl groups in BCN have an important influence on altering the band-gap structure, which results in increased production of reducing ·O2− radicals and fewer oxidizing ·OH radicals. Accordingly, the photoreduction efficiency of U(VI) by CCN in the carbonate (hydrogen) system is significantly boosted, 33 times higher compared to the raw g-C3N4.
The intrinsic van der Waals forces and π-π stacking between g-C3N4 layers often lead to severe aggregation, which enormously hinders the effective utilization of photo-generated charges and covers most of the active sites [83]. In response to this situation, Li et al. [99] adopted a morphology control strategy to prepare mesoporous g-C3N4 (MCNr). MCNr combines the advantages of porous structure and nanoscale, enhancing the specific surface area, and introducing appropriate surface defects and richer active sites, significantly improving exciton dissociation and visible light absorption range. Among them, MCN1.0 has the largest specific surface area, exhibiting the best photoreduction activity of U(VI) (Figure 4g–i).
Establishing donor-acceptor (D-A) structures is typically an effective strategy. To enhance π-electron transfer, Gong et al. [100] prepared a series of π-electron enhanced g-C3N4 by grafting aromatic ring donors. The photocatalytic activity of the modified sample was significantly enhanced in the photocatalytic elimination reaction of U(VI). Among them, g-C3N4 grafted with anthracene can remove almost all U(VI) within half an hour of visible light irradiation. In a subsequent study, Gong et al. [101] once again confirmed the effectiveness of the D-A method by incorporating thiophene as a donor into g-C3N4. While focusing on the performance of D-A type photocatalysts, Li et al. [54] systematically elucidated the extraction mechanism of uranium. The introduction of electron-rich carbon ring structure into g-C3N4 changed the morphology of the original material, reduced exciton dissociation energy, and promoted the adsorption and activation of O2. The abundant generation of reactive oxygen species greatly enhances the synthesis of hydrogen peroxide and immobilizes U(VI).
Conjugated microporous polymers (CMPs)
CMPs are organic conjugated microporous polymers linked by covalent bonds [102]. This emerging organic material has a semiconductor-like behavior and its unique designability is an ideal candidate for photocatalysts and shows outstanding characteristics compared to conventional semiconductors. The CMPs materials have large specific surface area, and high porosity, allow excellent exposure/accessibility of the substrate active site, and facilitate photogenerated charge/hole structure and chemical transport, making them ideal for various photocatalytic reactions [103–105]. Since it has many outstanding advantages, it is easy to combine it with the photocatalytic degradation of uranium (Table 2).
Summary of photocatalytic U(VI) based on CMP materials
The origins of conjugated polymer photocatalysts can be traced back to the 1980s when researchers in Japan conducted pioneering experiments showcasing the photocatalytic capabilities of poly(p-phenylene). They found that this polymer could initiate reactions when exposed to UV light, leading to the generation of small quantities of hydrogen in the presence of sacrificial agents [106]. Since then, the application of conjugated polymers has grown considerably and has shown its unique advantages in the field of photocatalysis.
In 2021, the group of Xia synthesized a novel conjugated microporous polymer (CMP) named TT-TPP through the aromatization reaction of CH. TT-TPP shows outstanding stability and photocatalytic properties. This material is capable of effectively catalyzing the reduction of U(VI) under highly acidic conditions (pH = 2–5) under visible light. TT-TPP demonstrates a high efficiency in removing U(VI), with a capacity of 3571.2 mg·g−1 [107]. Moreover, The CMPs known as pTTT-Ben were employed in the photoreduction of U(VI) under visible light. Operating under highly acidic conditions with a pH of 1, this material demonstrated exceptional U removal capabilities, achieving a high capacity of 4710 mg·g−1[108]. These studies have yielded some results in photocatalytic degradation of uranium, but their degradation is mediocre with long reaction times due to electron-hole recombination within the polymer and weak charge transfer within the molecule. How to effectively improve the efficiency of photocatalytic degradation of uranium by CMPs is still a critical issue. The mechanism based on the study of CMPs as photocatalysts can be described as light capture, separation, and transfer of photogenerated carriers, and redox reactions, while there are two primary avenues for enhancing photocatalytic performance: firstly, by focusing on bandgap engineering to optimize the processes of light absorption and charge separation; secondly, by leveraging bandgap engineering to enhance carrier mobility.
Additionally, drawing inspiration from the graded photosynthesis found in nature (Figure 2a), our team investigated a recently reported photocatalyst, ECUT-AQ structure comprises an electron-rich perylene unit as the donor and an electron-deficient anthraquinone (AQ) as the acceptor [109]. Notably, the donor-acceptor configuration of ECUT-AQ generates a significant built-in electric field, enhancing intramolecular charge transfer. This effect results in a considerable widening of the visible light absorption spectrum and an enhancement in the efficiency of separating carriers. The redox-active AQ in ECUT-AQ serves as a well-coordinated electron transfer pathway, facilitating the fast transfer of photogenerated electrons from the photocatalyst to UO22+. Accordingly, within a two-hour irradiation period, ECUT-AQ accomplished an 86% photoreduction of UO22+ and exhibited notable removal rate constants (k = 0.015 min−1, pH = 1 and T = 293.15 K), leading to enhanced degradation speed and capacity. Meanwhile, three novel full-spectrum (200 ≤ λ ≤ 800 nm) responsive biomimetic donor-acceptor conjugated microporous polymers (ECUT-BQ, ECUT-FQ, and ECUT-TQ) through a molecular engineering strategy [18]. Consequently, ECUT-TQ exhibits a narrow band gap as low as 1.70 eV with the strongest acceptor and enhanced built-in electric field, which achieved a high U(VI) removal rate of 97.4% with a k value of 0.057 min−1. Yang et al. [110] synthesized a family of pyrene-based nitrogen-implanted CMPs (PyCMP-0N, PyCMP-1N, PyCMP-2N, and PyCMP-3N), where the nitrogen gradient was regulated. As a result, PyCMP-3N with the highest BIEF intensity exhibited the optimal photocatalytic uranium extraction efficiency of 99.5%, which exceeded the control counterparts PyCMP-0N (41%), PyCMP-1N (71%), and PyCMP-2N (83%), respectively.
This study confirmed that by constructing CMPs with D-A structure, the molecular charge transfer within the conjugated polymers can be effectively promoted for better photocatalytic reduction of uranium. However, the D-A binary polymer also suffers from a fixed material feeding ratio and electron-hole complexation, which limits its photocatalytic ability. Therefore, an electron acceptor was incorporated into the D-A type conjugated microporous polymer to create a kind of D-A-A terpolymers through statistical copolymerization. Compared with D-A dimer copolymers, these D-A-A terpolymer CMPs exhibit a significant dipole moment and a substantial built-in electric field attributed to their multistage structure (Figure 2c), facilitating the separation of charge. Moreover, the terpolymer is polymerized from three components, which have a larger specific surface area and can enhance its capacity to absorb visible light and promote its photocatalytic degradation of U(VI) by changing its different molar feeding ratios. The conjugated microporous polymer catalyst synthesized by this method achieved 99.5% removal efficiency of U(VI) in 120 min under visible light, surpassing the majority of photocatalysts reported for such applications [111]. In general, the construction of D-A-A polymer systems can promote carrier mobility, i.e., the migration and transport of photogenerated electrons and holes, thus avoiding their complexation and extending the lifetime, which is closely related to photocatalytic performance. A pair of D-A CMPs for uranium photocatalytic reduction was developed by modulating skeleton isomerism through isomeric building blocks, named PQ-TPM (with phenanthrenequinone skeleton) and AQ-TPM (with anthraquinone skeleton) [112]. This strategy offers a more efficient method for enhancing CMP photocatalyst efficiency in treating radioactive wastewater.
Covalent organic framework (COFs)
The combination of U(VI) and a photocatalyst is a crucial factor for achieving efficient photoreduction performance. COFs are recognized as potential candidates for the photoreduction of radioactive elements due to extended π-conjugated layer structure, adjustable energy level, and chemical stabilities. In particular, the inherent regular porous network structures of COF materials can effectively combine uranium with photocatalysts and enhance photoreduction performance. Several studies have been conducted by simultaneously exploiting the adsorption and photocatalytic properties of COF materials (Table 3).
Summary of photocatalytic U(VI) based on COF materials
For example, the lamellar 2D TpBpy is synthesized by connecting a bipyridine unit (Bpy) which contains nitrogen-rich sites and can easily chelated with uranium to form bipyridyl salts [113]. The adsorption behavior of U(VI) towards TpBpy is suitable for a pseudo-second-order model, indicating that it involves chemisorption or surface complexation. The study of the photocatalytic performance of U(VI) showed that a 55.4% photocatalytic reduction efficiency was obtained under irradiation of visible light with a 350 W Xe lamp with a 420 nm cut-off filter as the light source. Considering the adsorption removal at the same time, the total removal rate of U(VI) can reach 76%. Thus, TpBpy exhibits significant synergistic chemical adsorption and photocatalytic reduction. The donor-acceptor covalent organic framework (COF) TTT–DTDA (TTT = thieno[3,2-b]thiophene-2,5-dicarbaldehyde and DTDA = 4,4′,4″-(1,3,5-triazine-2,4,6-triyl)trianiline) were evaluated photoreduction performances for U(VI) removal [114]. Donor-acceptor 2D COFs, with their outstanding charge carrier mobility and prolonged excited-state lifetimes, have become promising photochemistry materials. TTT–DTDA displayed a maximum absorption capacity of 123 mg U/g COF at pH = 5 after 10 h of irradiation in solutions lacking sacrificial reagents or protective gases. To delve deeper into the COF skeleton’s impact on photocatalytic activity, three β-keto-enamine COFs (Tp-Tapb, Tp-Taz, and TpTt) containing varying electron acceptors and the distances between the two acceptors were examined for their U(VI) adsorption and photoreduction capabilities. The results indicate that Tp-Taz and TpTt exhibit enhanced charge mobility efficiency and superior photocatalytic performance due to intramolecular D-A charge transfer (Figure 5a). Their adsorption capacities reached 338 and 505 mg·g−1. The enrichment of U(VI) facilitated photoreduction, leveraging the combined effects of adsorption and photoreduction. Consequently, the photocatalytic efficiencies of Tp-Taz and TpTt reached 50% and 55%, respectively, after 10 h of visible light exposure. TpTt outperformed Tp-Taz due to its short-range acceptor units containing a high proportion of heteroatoms .
 |
Figure 5 (a) The photoreduction mechanism of U(VI) over COFs skeleton , (b) LB-COF [115], and (c) Tp-DBD COF [116]. (d) Photochemical reduction and recycling experiment of PI-3-AR [117]. (e) DFT on diverse adsorption configurations and energetics for UO22+ binding to TpBD-X (001) and the elucidated rendering delineates the photocatalytically promoted U(VI) reaction mechanism [119]. |
However, the rapid recombination of photo-induced electron-hole pairs remains a significant challenge for COF materials’ catalytic performance. Xia et al. reported the synthesis of a pyrene-based amide COF, 4,4′,4″,4‴-(pyrene-1,3,6,8-tetrayl)tetrabenzaldehyde–3,8-diamino-6-phenylphenanthridine (TFPPy–DP), via postsynthetic modification of imine COFs [34]. These materials were utilized for the photoreduction of U(VI) under visible light. The incorporation of oxygen atoms into the framework and the alteration of polarity resulted in increased photo-induced electrons and a broader band gap, enhancing their hydrophilicity and dispersibility.
As such, imine COFs successfully achieved catalytic reduction equilibrium after 10 h of light exposure, achieving a remarkable 91% removal rate of 238 ppm U at pH = 3. However, amide COFs reached this equilibrium in just 2 h, demonstrating an impressive 82% removal rate. Zhang et al. meticulously elucidated the creation of a layer-blocked COF (LB-COF) film through two consecutive surface-initiated polycondensations, incorporating imine- and vinyl-linkages [115]. This LB-COF film showcases the excellent crystallinity and superior photoelectric activity of imine- and vinyl-linked COFs, respectively (Figure 5b). Consequently, LB-COF exhibits a remarkable photocatalytic reduction capacity for U(VI) at 320 mg·g−1, outperforming imine-linked COF (35 mg·g−1) and vinyl-linked COF (295 mg·g−1).
In recent years, COF materials have frequently been employed as photo-enhanced uranium adsorption agents due to their porous and photocatalytic properties. Qiu et al. shared the use of vinylene-linked COFs (Tp-TMT, Hb-TMT, and Tb-TMT) to boost adsorption performance via three synergistic mechanisms: selective ligand binding, photocatalytic reduction, and chemical reduction. Firstly, the abundance of hydroxyl groups in Tp-TMT acts as selective binding sites for U(VI). Secondly, these hydroxyl groups possess reducibility, capable of chemically reducing uranium in the dark. Lastly, the hydroxyl and triazine units in the conjugated framework synergistically decrease Tp-TMT’s band gap, enabling U(VI) photoreduction under illumination. As a result of these mechanisms, Tp-TMT’s adsorption capacity was reported to reach as high as 2362.4 mg·g−1 [116].
Cui et al. documented the development of ultra-stable benzoxazole-based COFs (Bd-DBD, Tp-DBD, and Hb-DBD) by integrating hydroxyl groups and benzoxazole rings. These COFs exhibited notable chemical reduction capabilities and a specific affinity for uranyl, facilitating the conversion of U(VI) to U(IV) [117]. In Figure 5c, the synergistic effect of hydroxyl groups and benzoxazole rings within the π-conjugated framework reduces the bandgap, thereby enhancing the photocatalytic efficiency of Bd-DBD, Tp-DBD, and Hb-DBD compared to Tb-DBD. This enhancement enables them to effectively combat major marine biofouling. Matthew et al. introduced a new amine-COF, PI-3-AR, derived from the reduction of the imine-linked COF, PI-3, using sodium borohydride (NaBH4). In Figure 5d, PI-3 was synthesized through solvothermal methods involving 1,3,5-triformyl benzene (TFB) and 4,4′,4″-(1,3,5-triazine-2,4,6-triyl)trianiline (TTA), aiming at effectively removing U(VI) from wastewater [118]. The PI-3-AR COF, along with a unique separation process involving amine-to-imine interconversion, proved effective in eliminating uranium, achieving a maximum adsorption capacity of 278 mg U/g COF and maintaining over 98% U(VI) recovery through five recycling at pH 4.0. Furthermore, PI-3-AR could be converted back to PI-3 through excessive use of oxidant iodine (I2) or the photoreduction of uranyl ions (UO22+). Moreover, modifying the backbone with functional groups exponentially enhances the affinity of U(VI) to COFs, while also regulating exciton dissociation and electron mobility for an ideal photoelectron transition pathway. Hu et al. elegantly demonstrated this by introducing varying donor-acceptor pairs within TpBD-COF (functional groups: –OH, –NH2, –OCH3, –NO2, –SO3H) [119]. The electron-withdrawing nature of –SO3H and –NO2, coupled with the electron-donating properties of –OH, –NH2, and –OCH3, allows for precise control over electron transfer between donors and acceptors and overall exciton dissociation energy (Figure 5e). Through IEF manipulation, there was a notable reduction in exciton dissociation energy from 105.39 MeV to 46.07 MeV, coupled with an enhancement in carrier lifetime from 1.076 ns to 3.109 ns. Furthermore, the N, O, and S atoms in various functional groups of TpBD-COF can act as π- and n-electron donors coordinating with U(VI), while intramolecular hydrogen bonds formed by these functional monomers facilitate photoexcited migration. Consequently, the uranium removal efficiency of TpBD-SO3H was significantly boosted by 5.5 times, demonstrating the synergistic enhancement in adsorption and photocatalytic performance.
Constructing a heterojunction structure acts as an effective strategy for extending the longevity of photogenerated electron-hole pairs. Qiu et al. presented a Z-type van der Waals heterojunction photocatalyst, SnS2COF, synthetized by amalgamating a COFs with a semiconductor (SnS2) (Figure 6a) [36]. In the absence of a protective atmosphere, the reduction efficiency of U(VI) of SnS2COF reached 1123.3 mg·g−1, surpassing that of SnS2 by 3.3 times and COF by 2.3 times. Additionally, a heterostructure of BiOBr@TpPa-1 was developed through in-situ COF growth on BiOBr 2D material to improve the lifetime of carriers for the photo-transformation of U(VI) to U(IV) and augment the U(VI) adsorption capacity. Photocatalytic reduction experiments were conducted under a 500 W Xenon lamp. The optimized BiOBr@TpPa-1 (5%) exhibited ultrahigh photoreduction efficiency of 91% within 450 min, compared to the degradation efficiency of bare BiOBr and TpPa-1 which were 65% and 82%, respectively. The higher removal rate could be accounted for rapid transfer and separation of excitons, which decreased the recombination rate of photo-induced electron-hole pairs due to the construction of II-type heterojunctions (Figure 6b). However, it is worth noting that the higher content of BiOBr in the BiOBr@TpPa (10%) affects the reduction rate of uranium because the high concentration of BiOBr can become the recombination centers of excitons, resulting in reduced photoactivity [51]. In light of the application environment for COFs under alkaline conditions (such as seawater, and alkaline uranium wastewater), it is worth noting that UO22+, positively charged in acidic environments, is dissimilar to UO2(CO3)3−, negatively charged, which displays weak affinity towards organic surfaces. This interaction can only occur through hydrogen bonding or physical adsorption, leading to a lack of an effective electron transfer pathway. To surmount this hurdle, Wang et al. introduced metallic substances into organic photocatalysts. Cationic metal sites can offer easy binding and reduction sites for UO2(CO3)3− [120]. Given that the dimensions of active metallic substances formed via conventional surface deposition and loading are so large that synergize effectively with organic components, TiOCs, as analogs of TiO2, are locally embedded in the micropores of COFs in the form of metal clusters. The confined TiOCs covalently bond at the molecular level with the COFs framework, enabling electrons to smoothly transfer from the organic donor part to the metal active catalytic sites (Figure 6c). This is also the first time that visible light catalytic reduction of UO2(CO3)3− in seawater has been achieved. Recently, Chen et al. have also proposed significant insights for the construction of COFs applied in alkaline wastewater. Unlike the photoredox process in traditional anaerobic environments, the use of porphyrin-Ni COF centers Ni2+ for O2 adsorption and photoredox can generate substantial hydrogen peroxide (H2O2) at the initial stage of the photocatalytic process [121]. In Figure 6d, a reasonable valence band (VB) can also oxidize H2O to O2, ensuring that under alkaline conditions, UO2(CO3)3− can bind with H2O2 to form an insoluble UO2(O2)2·2H2O solid without contacting the material surface, thereby achieving efficient separation.
 |
Figure 6 (a) The synthesis method and mechanism of U(VI) photoreduction in SnS2COF [36], and (b) BiOBr@TpPa-1 [51]. (c) Estimated energy levels of UO2(CO3)34−, COF-TZ, TiOCs, TiOCs⊂COF-TZ, and UO2(CO3)34−@TiOCs⊂COF-TZ [120]. (d) Mechanism of H2O2 photosynthesis and uranyl removal in TT-Por COF-Ni [121]. (e) Graphic view of the eclipsed AA stacking structure of Cu-TMT [118]. (f) The band structure diagram of Cu3(PyCA)3 and Cu-TMT [122]. |
Cui et al. described the synthesis of a C–C bonded metal-covalent organic framework named Cu-TMT through the Aldol condensation reaction (Figure 6e and f), utilizing the metal cluster Cu(PyCA) and 2,4,6-trimethyl-1,3,5-triazine (TMT) for photocatalytic removal of U(VI) [122]. The Cu clusters enclosed within well-ordered frameworks facilitate rapid transport pathways for charge transfer and effectively suppress photogenerated carrier recombination. This led to the reduction of U(VI) achieving 1438.8 mg·g−1 with a 98.1% removal efficiency for actual uranium-bearing sewage under visible light.
Recovering uranium from seawater and rare-earth tailings represents a significant area of research focus [123–125]. However, most of the COF sorbents/photocatalysts cannot achieve the goal of U(VI) extraction from seawater owing to the complex marine environment such as harsh ocean biological contamination, high concentration of competitive metal ions, and extremely low uranium concentration (~3.3 ppb). Amidoxime-functionalized COFs are considered a promising adsorbent to extract uranium from seawater due to their selective uranium adsorption, large specific surface area and excellent photocatalytic performance [123]. Qiu et al. synthesized naphthalene-based COF material NDA-TN-AO, containing amidoxime groups by using units with highly planar conjugated structures (Figure 7a). NDA-TN-AO showed not only improved antibacterial ability but also enhanced uranium adsorption capacity due to its outstanding photocatalytic activity. Under simulated sunlight irradiation, NDA-TN-AO’s adsorption performance from natural wastewater reached 6.07 mg·g−1, which was 1.3 times higher than that under dark conditions. Ma et al. introduced cooperative functions into the nanospace of COFs to achieve efficient extraction of uranium [124]. The COFs contain amidoxime groups that provide selective adsorption sites for uranium, while triazole units and bipyridyl functional groups achieve synergistic photocatalytic reduction and adsorption of U(VI) (Figure 7b). The best performing COF material they developed, 4-Pd-AO, achieved a high uranium removal rate of 4.62 mg·g−1 per day from seawater under visible light illumination. Mechanistic studies showed that 4-Pd-AO not only has excellent reduction and adsorption properties for uranium but generates 1O2 and ·O2−, achieving antibacterial and algal inhibition effects [125].
 |
Figure 7 (a) The synthesis of π-conjugated NDA-TN-AO and uranium extraction capacity [124]. (b) Synthetic scheme of COF 4 and COF 4-Pd-AO and U LIII-edge XANES spectra for COF 4-Pd-AO after uranium extraction studies [125]. (c) Uranyl adsorption kinetics on COF 2-Ru-AO at an initial uranium concentration of ~9 ppm, ~27 and 270 ppb in uranyl-spiked seawater, respectively. (d) Estimated energy levels of COF 2-Ru-AO [126]. |
The use of sacrificial reagents is usually necessary to improve the separation of photogenerated carriers in the photocatalytic process, but this also limits its practical applications. Wang et al. designed a range of ternary and quaternary COFs, they introduced bipyridine-5,5′-diamine-Ru(Bp)2]Cl2 as a secondary photosensitizer, enabling U(VI) extraction from seawater without sacrificial reagents. Notably, the quaternary COF 2-Ru-AO shows remarkable U(VI) extraction efficiency in the light, with enhanced production of the carrier pairs and photocatalytic performance without sacrificial agents. The extraction capacity of U(VI) reaches 7.36 mg·g−1 within 72 h from natural seawater (Figure 7c and d). These findings demonstrate the significant importance of adsorptive photocatalytic COF materials for uranium capture and lay the foundation for practical applications, such as seawater extraction [126].
Metal-organic framework (MOFs)
Compared to traditional inorganic semiconductor photocatalysts, COFs have technological advantages including many active sites, tunable structures, and the ease of transferring photo-generated electrons from metal orbitals to empty ligand orbitals under light irradiation. The excited electrons can reduce U(VI) to U(IV), which can achieve the purpose of immobilization or separation of uranium from water. Simultaneously, the photocatalytic activity of MOFs could be optimized by selecting suitable metal nodes and chromophoric ligands. Therefore, MOF materials have received great attention in the photocatalytic reduction of heavy/radioactive metal(loid)s, especially in the photocatalytic enhanced adsorption and removal of uranium, which has been widely reported. The mixed-linker strategy is an effective way to enhance photoreduction-assisted uranium recovery by MOFs (Table 4).
Summary of photocatalytic U(VI) based on MOF materials
For instance, Wang et al. [127] created organic framework material (SCU-19) that showcases an unusual inclination that has been meticulously designed and synthesized, employing the solvent-thermal method. Under darkness, SCU-19 can efficiently capture uranium via ligand coordination using its exposed oxygen atoms, and some chemical reduction from U(VI) to U(IV) by lower valence Mo atoms in POM. Under visible light irradiation, it triggers a reducing mechanism; resulting in a higher uranium removal rate, without saturation, and faster adsorption kinetics. Thus, SCU-19 is the sole uranium adsorbent with three distinct adsorption mechanisms. Wang et al. achieved the incorporation of a photo-responsive Ni(II) porphyrin (TCPP(Ni)) ligand into UIO-66-NH2 (TCPP(Ni)⊂MOF) via a one-pot synthesis method, while preserving the crystal structure and exceptional chemical stability (Figure 8a). Following optimization, TCPP(Ni)⊂ MOF-3 displayed remarkable selectivity in capturing U(VI) from wastewater or seawater, employing a combination of complexation and photo-reduction. Notably, even after immersion in natural seawater for 25 days, it maintained a high capacity to adsorb U(VI) of 8.95±0.39 mg-U/g-Ads, and it also demonstrated good antibacterial properties [128]. In addition to the mixed-linker strategy, post-synthetically modified photoactive COFs through combined ligand complexation and photoreduction is also an effective way to extract uranium. PN-PCN-222 is synthesized by incorporating phosphorous and amino groups as ligands into the photoactive MOF PCN-222. The photogenerated electron electrons produced by PN-PCN-222 under visible light can effectively reduce the U(VI) pre-concentrated in MOF to U(IV), which is then separated from the PN-PCN-222 and regenerated active sites for capturing additional U(VI). Under light irradiation, the photogenerated electrons from PN-PCN-222 efficiently convert pre-concentrated U(VI) in the MOF to U(IV). Subsequently, the U(IV) is separated from PN-PCN-222, allowing for the regeneration of active sites to capture additional U(VI). Under the photoreduction of PN-PCN-222, the adsorption capacity and extraction efficiency reach 756.1 mg·g−1 and 96.7%, as the initial concentration of U(VI) is 400 ppm. More importantly, PN-PCN-222 could be used for U(VI) separation over a wide range of U(VI) concentrations and pH values, demonstrating its strong potential for practical applications [129].
 |
Figure 8 (a) Schematic illustration of the proposed mechanism on photocatalytic reduction of U(VI) for TCPP(Ni)⊂MOF-3 [128], and (b) NZVI@PCN-224 [130]. (c) Stability and antibacterial activity of BP@CNF-MOF after exposure to natural seawater for 0, 15, and 30 days [133]. (d) Proposed mechanism on photocatalytic reduction of U(VI) for Ti-MOF@DATp [134], (e) POMOF and (f) Cu@Th-TCPP [136]. (g) SEM images (scale bar, 5 μm) of the AOP@ZIF-8/TA fibers after each elution [137]. |
Building heterojunctions widens the visible light absorption range and facilitates the rapid separation of photogenerated carriers, enhancing the photocatalytic reduction of uranium. Wu et al. successfully fabricated a regenerable antibiofouling heterojunction system based on a porphyrinic zirconium metal-organic framework doped with nano zero-valent iron (NZVI@PCN-224) [130]. The photocatalytic removal of uranium could be improved by immobilizing NZVI nanomaterials on PCN-224, owing to the Schottky-junction effect on the interface of NZVI/MOF enhances mobility and separation of interfacial electrons. Additionally, the photocatalytic effect of NZVI@PCN-224 generates a large number of biotoxic ROS, resulting in stronger antibacterial and antialgal effects (Figure 8b). Under simulated sunlight, NZVI@PCN-224 shows its highest adsorption capacity of 57.94 mg·g−1 at pH = 4.0, significantly surpassing its adsorption capacity under dark conditions (approximately 28% at pH = 5.0). Even after eight cycles, NZVI@PCN-224 maintained a remarkable adsorption capacity of U(VI) of 47.98 mg·g−1 and a removal rate of 91.72%. The strategy of integrating other metal atoms with MOFs can be utilized to create novel photocatalytic heterojunctions, offering a novel method for the photocatalytic treatment of uranium-containing wastewater.
In addition to being constructed with metals, COFs have also been used in combination with black phosphorus quantum dots to construct heterojunction structures [131]. Highly porous carboxyl cellulose nanofiber (CNF) aerogels are utilized to anchor UiO-66-NH2/black phosphorus quantum dots (MOF/BPQDs) heterojunctions, creating high-efficiency U(VI) adsorbents (BP@CNF-MOF). Because of the mutual physical interactions and entanglements of CNFs, along with strong binding interactions between MOF crystals and CNF aerogel, BP@CNF-MOF exhibits excellent mechanical flexibility and stability (Figure 8c). The outstanding photocatalytic performance of MOF/BPQDs enables the effective eradication of marine bacteria via ROS and facilitates the degradation efficiency of U(VI), thereby creating additional binding sites on MOF crystals to further adsorb U(VI). Under visible light, the adsorption efficiency of U(VI) of BP@CNF-MOF increased by 55.3% compared to dark conditions, reaching 6.77 mg-U per g-Ads after light irradiation for 6 weeks. The MOF coating layer also helped to stabilize the BPQDs.
The above introduces that the two materials are heterojunction structures formed by organic MOFs and inorganic elements. Considering the compatibility issues of heterojunctions, Hu et al. utilized two common MOF photocatalysts (MOF-In, MOF-Ti) and the assembled heterojunctions MOF@COF (NH2-MIL-125(Ti)@ TpPa-1) for the photocatalytic reduction of U(VI) [132]. The findings revealed that degradation efficiency of U(VI) were 55.6% for NH2-MIL-68(In), 57.7% for NH2-MIL-125(Ti), and 81.6% for NH2-MIL-125(Ti)@ TpPa-1. The presence of unsaturated coordination (active Ti3+ and oxygen vacancies) in MOF can effectively promote the generation of superoxide radicals and suppress the recombination of photogenerated electron-hole pairs. The study of NH2-MIL-125(Ti)@TpPa-1 confirms the synergistic role of MOF@COF hybridization in adsorption-photocatalytic reduction, exhibiting promising prospects for U(VI) removal across various applications.
Qiu et al. demonstrated the construction of Ti-MOF@DATp, where a metal-organic framework (Ti-MOF) serves as the core with COFs (DATp) shells grown in-situ on its surface, enabling photoreduction of U(VI) and degradation of tetracycline [133]. The staggered energy levels between Ti-MOF and DATp form a Z-Scheme heterojunction via covalent bonds, facilitating carrier transfer and enhancing photocatalytic activity (Figure 8d). Consequently, Ti-MOF@DATp achieves a photocatalytic removal rate of 96% U(VI) and 90% tetracycline from a mixed solution. Liu et al. employed a new POMOF, [Cu(4,4′-bipy)]5·{AsMo4VMo6VIV2VO40(VIVO)[VIVO(H2O)]}·2H2O (1), achieving a remarkable removal rate of U(VI) of 99% [134]. The exceptional photoreduction of U(VI) by this POMOF catalyst stems mainly from the synergistic interaction between Cu(I)–MOFs and reduced {AsMo10V4} clusters (Figure 8e).
In addition to the common methods mentioned above to improve the photocatalytic efficiency of MOFs materials, defect engineering, integration with organic oxidation, coatings with dynamic surfaces, ions-induced framework interpenetration, and other methods are also often used. Wang et al. reported the application of missing-linker defects and node metal substitution in UiO-66-NH2 (Zr-MOF) to enhance its photocatalytic properties of U(VI). The absence of linker molecules leads to the formation of additional defects, resulting in more porous frameworks, which enhances the rapid diffusion of U(VI) into the inner pores and boosts the utilization of adsorption binding sites within the material [135]. Luo et al. reported the integration of photoreduction and chemical reduction into a new extended π-conjugated framework (Cu@Th-TCPP) for photocatalytic uranium extraction without the expense of sacrificial reagents (Figure 8g) [136]. Wu’s group developed a new type of coating that utilizes the material’s photocatalytic properties, endowing the material with durability and outstanding anti-fouling capabilities. The coating comprises a self-polishing hybrid nanofibrous mat of zeolitic imidazolate framework-8 (ZIF-8) and amidoximed polyacrylonitrile (AOP), known as AOP@ZIF-8/TA, which is cross-linked with hydrolyzable tannic acid (TA) to generate a dynamic surface (Figure 8g) [137]. Current issues facing MOF materials include insufficient stability, poor spectral matching, limited adsorption capacity, high synthesis cost, the presence of metal ions that may cause secondary pollution, and poor repeatability. These problems will still need to be improved and researched in the future.
DISSECT OF URANIUM REDUCTION PRODUCTS
The experimental photoreduction of uranium can be divided into two different reaction conditions: inert atmosphere and air atmosphere. The reduction products of uranium under different atmospheres are generally different, including UO2, (UO2)O2·2H2O, U3O8, and UO3. Accordingly, it is essential to determine the intermediates and reduction mechanisms to elucidate the mechanism of photoreduction. The characterization of uranium reduction products mainly includes XRD, SEM, TEM, XPS, and XAFS, which are used to determine the crystal structure, morphology, and valence state of the reduction products, respectively. The characterization and experiments of the active substances in the photoreduction process are analyzed using EPR and free radical trapping experiments.
Inert atmosphere
The experiments on photocatalytic reduction of U(VI) were mainly conducted under an inert atmosphere, primarily an N2 atmosphere. Before the photocatalytic reaction, oxygen in the reactor and uranium solution is removed by vacuuming or purging with nitrogen to prevent the reduced uranium from being oxidized again. Furthermore, dissolved oxygen also competes with uranium for photo-generated electrons, thereby impeding the reduction process of uranium. For example, Salomone et al. employed TiO2 as a photocatalyst and 2-propanol as a hole scavenger [138]. They conducted photoreduction of U(VI) under different atmospheres, including air, oxygen-bubbled, and nitrogen-bubbled, achieving uranium removal efficiencies of 66%, 12%, and 73%, respectively. Moreover, under the presence of dissolved oxygen, the reduction of uranium oxide (UO2+x) on the surface of TiO2 was inhibited. UO2+x is a uranium oxide composed of U(VI) and U(IV), formed by the interaction between uranyl ions and photo-generated electrons. Lu et al. investigated the photocatalytic performance under oxygen and nitrogen atmospheres using boron-doped C3N4. The findings indicated a lower photocatalytic rate under an oxygen atmosphere compared to a nitrogen atmosphere, yet still achieving a removal rate of 70%. To obtain a deeper insight into the valence state and morphology of the products generated during photocatalytic reduction, Li et al. used XRD to determine that the reduced product after photocatalysis was U3O7. XANES analysis determined that the uranium absorption edge was located between UO2 and UO2(OH)2, and XPS confirmed that the proportion of U(IV) was 22.1% while U(VI) was 87.9%, consistent with the conclusions of XRD and XANES. However, some studies have reported amorphous UO2 as the reduction product. This is mainly due to the strong adsorption interaction between the active sites on the material’s surface and the uranyl ions before photocatalysis, which hinders the growth of UO2 crystals [139]. Alternatively, it may be because the nucleation rate of UO2 is too fast, resulting in the formation of a large amount of amorphous UO2. Furthermore, Li’s groups introduced a unique post-synthetically functionalized metal-organic framework (PCN-222) into the uranium capture process [129]. The photocatalytic reduction of uranium led to XRD peaks in addition to the original ones, which perfectly aligned with (UO2)O2·2H2O (JCPDS 01-081-9033). Similarly, Qiu’s research team observed a pinpointed deposition of UO2 within TEM images [35].
Air atmosphere
To promote practical applications of photocatalytic reduction of uranium, most experiments are currently conducted under air atmosphere. Under these conditions, both uranyl ions and dissolved oxygen, two substances that can easily accept electrons, coexist in the solution. Therefore, the competitive electron-accepting process of dissolved oxygen cannot be ignored. The oxygen reduction reaction mainly involves the one-electron reduction to generate superoxide radicals (·O2−) and the two-electron reduction to generate H2O2. Wang et al. synthesized graphene aerogel (GA) for adsorption-photocatalytic extraction of uranium [140]. SEM revealed the presence of numerous rod-shaped uranium oxides on the surface of GA after photocatalytic reaction. XRD analysis confirmed that the uranium oxide was metastudtite (UO2)O2·2H2O. Li et al. employed Raman spectroscopy to investigate the formation process of (UO2)O2·2H2O. These findings indicated that (UO2)O2·2H2O can only form under atmospheric conditions, and is produced through the reaction of UO2 with H2O2. Moreover, the (UO2)O2·2H2O can also be generated through the reaction of UO2 with ·O2−. Li et al. synthesized a CdS/UiO-66-NH2 heterojunction photocatalyst for the treatment of uranium-bearing sewage from mines [78]. The mechanism investigation revealed that ·O2− and photogenerated electrons are the main active species, and there is no generation of H2O2 during the photocatalytic reaction. Ultimately, the reduction product was confirmed to be amorphous (UO2)O2·xH2O, which was generated by the reaction between ·O2− and UO2, as evidenced by Raman spectroscopy and XRD analysis. Under atmospheric conditions, other uranium reduction products such as U3O8 and UO3·NH3 are also generated. Dong et al. synthesized three types of CdS/TiO2-based hollow sphere photocatalysts and demonstrated through Raman spectroscopy that the photocatalytic reduction products were all U3O8 [32,141,142]. Free radical capture experiments showed that the addition of P-BQ (·O2− scavenger) and K2Cr2O7 (photo-electron scavenger) suppressed the photocatalytic reaction, indicating that the main active species were ·O2− and photogenerated electrons, and that U3O8 was formed by the re-oxidation reaction between UO2 and ·O2−. It can be seen that how dissolved oxygen is reduced has a significant influence on the formation of uranium reduction products. Liang and Gong, unusually, proved the existence of UO3·NH3 in uranium reduction products through XRD, but the formation mechanism has not been deeply explored [143]. NH3 is certainly formed during the photocatalytic reaction, but its origin is still uncertain. The N2 could originate from the reduction of nitrate or the reduction of N2. When uranium dioxide and ammonia water are present in a solution, UO3·NH3 does not bind even when great quantities of ammonia are present. Conversely, when insoluble solid impurities are also present, they serve as nucleation sites for the formation of UO3·NH3. Then, the coordination reaction between the uranyl ion and ammonia water arises, leading to the creation of UO3·NH3. When NH3 is produced during the photoreaction, the photocatalyst can act as a nucleation site for this coordination reaction.
CHALLENGES AND PERSPECTIVES IN THE PHOTOREDUCTION OF U(VI)
Although the photocatalytic efficiency for U(VI) reduction in polymers is remarkable, the connection between exciton behavior and photocatalytic efficacy requires further exploration. Consequently, in comparison to well-known mechanisms of U(VI) photoreduction, the U(VI) elimination via photocatalysis in a polymer’s natural environment requires closer attention, revealing a multitude of challenges that remain to be tackled.
For the whole society, the decontamination of uranium-containing sewage demands photocatalysts that are both energy-efficient and economically feasible, while effectively removing pollutants. Despite the energy efficiency and utilization of eco-friendly visible light in polymeric photocatalysts for pollutant removal, their widespread adoption in society is hindered by the high cost of synthesis. The integration of custom-designed organic monomers into the backbone of polymeric photocatalysts is essential for their photoactivity. However, the synthesis of these moieties typically involves complex multistep reactions, leading to increased production costs. Additionally, the moderate yields of most polymeric photocatalysts contribute to their elevated synthetic expenses. It remains challenging to precisely determine how structural elements influence the photoreduction of U(VI) in polymeric photocatalysts. Particularly in CMPs, their disordered and varied structures have heated debate, contrasting sharply with the fixed structures of inorganic crystals. The inherent flexibility of polymers complicates the selective incorporation of specific structural features. Accordingly, developing and characterizing well-defined polymers are critical for exploring the links between their structures and photoreduction activities. Recent researches are noteworthy: synthetic methods like Knoevenagel condensation, Aldol condensation, and Horner-Wadsworth-Emmons reaction are instrumental in crafting precisely defined polymers. Meanwhile, methods such as synchrotron radiation-based spectroscopy and electrostatic force microscopy are proving effective for analyzing the intricate structures and electronic characteristics of these materials.
Additionally, the adsorption of uranyl ions on materials’ surfaces is vital in the photocatalytic reduction of uranium. Here, the coordination geometry, reaction species, and environmental factors can greatly affect efficiency and selectivity. To solve these problems, it is necessary to monitor the adsorption, desorption, and activation of uranyl photocatalytic reduction on the surface of the photocatalyst. For example, in-situ FT-IR and XPS can monitor reactive sites and coordination geometries. Furthermore, control experiments under specific external fields aid in understanding excitonic behaviors in polymeric photocatalysts. For instance, magnetic-field-dependent steady-state and time-resolved photoluminescence tests can reveal the energy levels and kinetics of excitons with different spin multiplicities. Electric-field-dependent tests help investigate the properties of charged excitons and dark excitons. Space-resolved spectroscopic analyses are beneficial for visually discerning excitonic properties such as transport and quenching in polymer-based materials. To boost photo-induced charge separation, DFT-based, frontier molecular orbital, and electronic structure calculations for charge mobility can be utilized to modify appropriately the HOMO-LUMO levels in polymer-based materials.
Expanding the scope of photocatalytic efficiency in the U(VI) transfer pathway during photoreduction can be challenging. Moreover, regarding uranium photoreduction, the reduction route of U(VI) remains contentious, including electron reduction, disproportionation of U(V), and radical reduction. With the recent advances in high-end spectroscopic and microscopic tools, prominent advancements have been made with in situ TEM, XPS, EPR, and XAFS techniques. These methods offer promising prospects for identifying intermediate uranium species during photocatalytic uranium reduction. Moreover, in situ EPR, XPS, and XAFS for uranium photoreduction can track the coordination structure evolution of uranium compounds on semiconductors on a temporal scale, which is crucial for understanding the uranium photoreduction mechanism.
Optimizing the structural integrity and sustainability of materials is paramount in seawater U(VI) extraction engineering, serving as a pivotal factor in enhancing the financial efficiency of this process. Utilization of the existing infrastructure is essential and a thorough assessment of seawater U(VI) extraction economics, complemented by comprehensive seawater trials at a considerable scale, can significantly elevate stability levels. The material’s inherent resilience should not be overlooked, particularly in terms of (1) its resistance against biofouling; (2) U(VI) elution efficacy; and (3) reusability of U(VI). Addressing the issues surrounding seawater U(VI) extraction requires the implementation of strategies that utilize moderate synthesis methods, finely tune the reaction and regeneration processes, and build a robust framework. This approach requires significant innovation and the development of novel technologies, including those proposed in the form of MOFs and COFs, which demonstrate exceptional performance but also carry substantial costs and stringent synthetic prerequisites that hinder widespread use. Currently, available photocatalyst technologies predominantly produce porous powders that do not satisfy the criteria for effective seawater extraction. Therefore, the engineering of a macroscopic photocatalytic integrated system, equipped with mechanical attributes, represents a significant challenge that must be overcome to achieve practical uranium extraction from seawater.
CONCLUSIONS
To tackle these challenges, future research should concentrate on devising a cost-effective and efficient method for manufacturing polymeric photocatalysts. These structures adeptly generate various ROS like singlet oxygen (1O2), ·O2−, H2O2, etc., upon illumination, expediting the swift remediation of U(VI) in radioactive wastewater. Nonetheless, conducting a thorough post-catalytic analysis of the species is vital, considering potential structural changes during the reaction and consequent alterations in electronic properties. Bound states for charge separation can exhibit similar activity as long as the substrate’s redox potential aligns with the exciton band potential. However, the lack of adequate analytical and instrumentation tools for monitoring exciton functionality in probe photocatalysis makes it challenging to precisely assess the relative influence of bound versus mobile charges. Furthermore, utilizing density functional theory for analyzing mechanistic and theoretical research calculations could yield crucial insights into this material class. A comprehensive understanding of its underlying dynamics and mechanisms requires precise comprehension from the molecular to macroscopic levels. Consequently, studying polymeric photocatalysts post-water treatment and formulating guidelines for their practical application in radioactive wastewater is imperative. While much remains to be understood, with the right tools at our disposal, the future of this field holds great promise.
Data availability
The original data are available from corresponding authors upon reasonable request.
Acknowledgments
We are grateful to the participants for donating the blood samples and data for this study.
Funding
This work was supported by the National Natural Science Foundation of China (22206024, 22276030, U2167223 and 2237061129), and the Jiangxi Provincial Natural Science Foundation (20232BAB213034 and 20232ACB203011).
Author contributions
Z.D. conducted the investigation and wrote the original draft. J.C. was responsible for the investigation and data curation. C.L., Z.L., J.H., Y.W., Z.L., and F.Y. contributed to data curation, software development, and validation. Z.Z. and Y.L. oversaw project administration, secured funding, and performed validation and supervision. All authors have reviewed and consented to the final version of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Ikemoto I. Nuclear power as an energy source. Energy Resources 1999; 20: 367–368 [Google Scholar]
- Skinner LB, Benmore CJ, Weber JKR, et al. Molten uranium dioxide structure and dynamics. Science 2014; 346: 984-987. [Article] [CrossRef] [PubMed] [Google Scholar]
- Wang D, Song J, Lin S, et al. A marine‐inspired hybrid sponge for highly efficient uranium extraction from seawater. Adv Funct Mater 2019; 29: 1901009. [Article] [CrossRef] [Google Scholar]
- Bourrachot S, Brion F, Pereira S, et al. Effects of depleted uranium on the reproductive success and F1 generation survival of zebrafish (Danio rerio). Aquat Toxicol 2014; 154: 1-11. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Chen Y, Yin X, Zheng N, et al. Flexible self-supporting Na3MnTi(PO4)3@C fibers for uranium extraction from seawater by electro sorption. J Hazard Mater 2024; 461: 132664. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Wang D, Xu Y, Xiao D, et al. Ultra-thin iron phosphate nanosheets for high efficient U(VI) adsorption. J Hazard Mater 2019; 371: 83-93. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Zhang Y, Mei B, Tian X, et al. Remediation of uranium(VI)-containing wastewater based on a novel graphene oxide/hydroxyapatite membrane. J Membr Sci 2023; 675: 121543. [Article] [Google Scholar]
- Zhou L, Li Y, Shao Y, et al. Interface coupling induced built-in electric fields accelerate electro-assisted uranium extraction over Co3O4@FeOx nanosheet arrays. Appl Catal B-Environ Energy 2024; 353: 124052. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Satpathy A, Catalano JG, Giammar DE. Reduction of U(VI) on chemically reduced montmorillonite and surface complexation modeling of adsorbed U(IV). Environ Sci Technol 2022; 56: 4111-4120. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Dong Z, Gao D, Li Z, et al. Harvesting the vibration energy of CdS for high‐efficient piezo‐photocatalysis removal of U(VI): Roles of shape dependent and piezoelectric polarization. Energy Environ Mater 2024; 7: e12705. [Article] [CrossRef] [Google Scholar]
- Dong Z, Zhang Z, Wang T, et al. Ingenious design of ternary hollow nanosphere with shell hierarchical tandem heterojunctions toward optimized visible-light photocatalytic reduction of U(VI). Sep Purif Technol 2022; 286: 120418. [Article] [CrossRef] [Google Scholar]
- Amadelli R, Maldotti A, Sostero S, et al. Photodeposition of uranium oxides onto TiO2 from aqueous uranyl solutions. Faraday Trans 1991; 87: 3267-3273. [Article] [CrossRef] [Google Scholar]
- Meng Q, Wu L, Yang X, et al. Photo-enhanced uranium recovery from spent fuel reprocessing wastewater via S-scheme 2D/0D C3N5/Fe2O3 heterojunctions. SusMat 2024; 4: e199. [Article] [CrossRef] [Google Scholar]
- Yu K, Li Y, Cao X, et al. In-situ constructing amidoxime groups on metal-free g-C3N4 to enhance chemisorption, light absorption, and carrier separation for efficient photo-assisted uranium(VI) extraction. J Hazard Mater 2023; 460: 132356. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Yang G, Wei L, Wang X, et al. Enhancing commercially iron powder electron transport by surface biosulfuration to achieve uranium extraction from uranium ore wastewater. Inorg Chem 2024; 63: 1378-1387. [Article] [CrossRef] [PubMed] [Google Scholar]
- Liu Z, Yao S, Zhang A, et al. Intramolecular built-in electric field enhanced polymerized nitrogen-carbon homojunction π*-electron delocalization enrichment promotes photocatalytic uranium (VI) reduction. Appl Catal B-Environ 2023; 338: 123023. [Article] [Google Scholar]
- Zhang W, Li L, Gao Y, et al. Graphitic carbon nitride-based materials for photocatalytic reduction of U(VI). New J Chem 2020; 44: 19961-19976. [Article] [CrossRef] [Google Scholar]
- Yu F, Yu S, Li C, et al. Molecular engineering of biomimetic donor-acceptor conjugated microporous polymers with full-spectrum response and an unusual electronic shuttle for enhanced uranium(VI) photoreduction. Chem Eng J 2023; 466: 143285. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Zhong X, Ling Q, Ren Z, et al. Immobilization of U(VI) onto covalent organic frameworks with the different periodic structure by photocatalytic reduction. Appl Catal B-Environ 2023; 326: 122398. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Feng L, Yuan Y, Yan B, et al. Halogen hydrogen-bonded organic framework (XHOF) constructed by singlet open-shell diradical for efficient photoreduction of U(VI). Nat Commun 2022; 13: 1389. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Chen W, Cai Y, Lv Z, et al. Improvement of U(VI) removal by tuning magnetic metal organic frameworks with amine ligands. J Mol Liq 2021; 334: 116495. [Article] [CrossRef] [Google Scholar]
- Wu P, Yin X, Zhao Y, et al. Porphyrin-based hydrogen-bonded organic framework for visible light driven photocatalytic removal of U(VI) from real low-level radioactive wastewater. J Hazard Mater 2023; 459: 132179. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Sprick RS, Jiang JX, Bonillo B, et al. Tunable organic photocatalysts for visible-light-driven hydrogen evolution. J Am Chem Soc 2015; 137: 3265-3270. [Article] [Google Scholar]
- Yang C, Ma BC, Zhang L, et al. Molecular engineering of conjugated polybenzothiadiazoles for enhanced hydrogen production by photosynthesis. Angew Chem Int Ed 2016; 55: 9202-9206. [Article] [CrossRef] [PubMed] [Google Scholar]
- Yu F, Zhu Z, Wang S, et al. Tunable perylene-based donor-acceptor conjugated microporous polymer to significantly enhance photocatalytic uranium extraction from seawater. Chem Eng J 2021; 412: 127558. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Wang Y, Chen G, Weng H, et al. Carbon-doped boron nitride nanosheets with adjustable band structure for efficient photocatalytic U(VI) reduction under visible light. Chem Eng J 2021; 410: 128280. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Wang J, Li P, Wang Y, et al. New strategy for the persistent photocatalytic reduction of U(VI): Utilization and storage of solar energy in K+ and cyano co‐decorated poly(heptazine imide). Adv Sci 2023; 10: e2205542. [Article] [CrossRef] [Google Scholar]
- Ren W, Cheng J, Ou H, et al. Enhancing visible‐light hydrogen evolution performance of crystalline carbon nitride by defect engineering. ChemSusChem 2019; 12: 3257-3262. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Dai Z, Zhao S, Lian J, et al. Efficient visible-light-driven photoreduction of U(VI) by carbon dots modified porous g-C3N4. Sep Purif Technol 2022; 298: 121590. [Article] [Google Scholar]
- Kim H, Kwon OS, Kim S, et al. Harnessing low energy photons (635 nm) for the production of H2O2 using upconversion nanohybrid photocatalysts. Energy Environ Sci 2016; 9: 1063-1073. [Article] [Google Scholar]
- Chen X, Zhang W, Zhang L, et al. Sacrificial agent-free photocatalytic H2O2 evolution via two-electron oxygen reduction using a ternary α-Fe2O3/CQD@g-C3N4 photocatalyst with broad-spectrum response. J Mater Chem A 2020; 8: 18816-18825. [Article] [Google Scholar]
- Dong Z, Meng C, Li Z, et al. Novel Co3O4@TiO2@CdS@Au double-shelled nanocage for high-efficient photocatalysis removal of U(VI): Roles of spatial charges separation and photothermal effect. J Hazard Mater 2023; 452: 131248. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Tu B, Yu K, Fu D, et al. Amino-rich Ag-NWs/NH2-MIL-125(Ti) hybrid heterostructure via LSPR effect for photo-assist uranium extraction from fluorine-containing uranium wastewater without sacrificial agents. Appl Catal B-Environ 2023; 337: 122965. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Kang J, Hang J, Chen B, et al. Amide linkages in pyrene-based covalent organic frameworks toward efficient photocatalytic reduction of uranyl. ACS Appl Mater Interfaces 2022; 14: 57225-57234. [Article] [Google Scholar]
- Xu RH, Cui WR, Zhang CR, et al. Vinylene-linked covalent organic frameworks with enhanced uranium adsorption through three synergistic mechanisms. Chem Eng J 2021; 419: 129550. [Article] [Google Scholar]
- Liu X, Bi RX, Zhang CR, et al. SnS2-covalent organic framework Z-scheme van der Waals heterojunction for enhanced photocatalytic reduction of uranium (VI) in rare earth tailings wastewater. Chem Eng J 2023; 460: 141756. [Article] [CrossRef] [Google Scholar]
- Fu J, Zhu B, Jiang C, et al. Hierarchical porous O‐doped g‐C3N4 with enhanced photocatalytic CO2 reduction activity. Small 2017; 13: 1603938. [Article] [CrossRef] [Google Scholar]
- Zhang G, Ou W, Wang J, et al. Stable, carrier separation tailorable conjugated microporous polymers as a platform for highly efficient photocatalytic H2 evolution. Appl Catal B-Environ 2019; 245: 114-121. [Article] [Google Scholar]
- Sheng J, Dong H, Meng X, et al. Effect of different functional groups on photocatalytic hydrogen evolution in covalent‐organic frameworks. ChemCatChem 2019; 11: 2313-2319. [Article] [CrossRef] [Google Scholar]
- Chen J, Dong CL, Zhao D, et al. Molecular design of polymer heterojunctions for efficient solar–hydrogen conversion. Adv Mater 2017; 29: 1606198. [Article] [CrossRef] [Google Scholar]
- Zhang Q, Kelly MA, Bauer N, et al. The curious case of fluorination of conjugated polymers for solar cells. Acc Chem Res 2017; 50: 2401-2409. [Article] [Google Scholar]
- Zhong W, Liang J, Hu S, et al. Effect of monofluoro substitution on the optoelectronic properties of benzo[c][1,2,5]thiadiazole based organic semiconductors. Macromolecules 2016; 49: 5806-5816. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Xiang Y, Wang X, Rao L, et al. Conjugated polymers with sequential fluorination for enhanced photocatalytic H2 evolution via proton-coupled electron transfer. ACS Energy Lett 2018; 3: 2544-2549. [Article] [Google Scholar]
- Cheng Z, Fang W, Zhao T, et al. Efficient visible-light-driven photocatalytic hydrogen evolution on phosphorus-doped covalent triazine-based frameworks. ACS Appl Mater Interfaces 2018; 10: 41415-41421. [Article] [Google Scholar]
- Vyas VS, Haase F, Stegbauer L, et al. A tunable azine covalent organic framework platform for visible light-induced hydrogen generation. Nat Commun 2015; 6: 8508. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Pachfule P, Acharjya A, Roeser J, et al. Diacetylene functionalized covalent organic framework (COF) for photocatalytic hydrogen generation. J Am Chem Soc 2018; 140: 1423-1427. [Article] [CrossRef] [PubMed] [Google Scholar]
- Jin E, Lan Z, Jiang Q, et al. 2D sp2 carbon-conjugated covalent organic frameworks for photocatalytic hydrogen production from water. Chem 2019; 5: 1632-1647. [Article] [Google Scholar]
- He S, Yang Z, Cui X, et al. Fabrication of the novel Ag-doped SnS2@InVO4 composite with high adsorption-photocatalysis for the removal of uranium (VI). Chemosphere 2020; 260: 127548. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Li S, Yang P, Liu X, et al. Graphene oxide based dopamine mussel-like cross-linked polyethylene imine nanocomposite coating with enhanced hexavalent uranium adsorption. J Mater Chem A 2019; 7: 16902-16911. [Article] [Google Scholar]
- Liu C, Dong Z, Yu C, et al. Study on photocatalytic performance of hexagonal SnS2/g-C3N4 nanosheets and its application to reduce U (VI) in sunlight. Appl Surf Sci 2021; 537: 147754. [Article] [Google Scholar]
- Zhong X, Liu Y, Wang S, et al. In-situ growth of COF on BiOBr 2D material with excellent visible-light-responsive activity for U(VI) photocatalytic reduction. Sep Purif Technol 2021; 279: 119627. [Article] [Google Scholar]
- Li P, Wang J, Wang Y, et al. Ultrafast recovery of aqueous uranium: Photocatalytic U(VI) reduction over CdS/g-C3N4. Chem Eng J 2021; 425: 131552. [Article] [CrossRef] [Google Scholar]
- Meng Q, Yang X, Wu L, et al. Metal-free 2D/2D C3N5/GO nanosheets with customized energy-level structure for radioactive nuclear wastewater treatment. J Hazard Mater 2022; 422: 126912. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Zhang Z, Liu C, Dong Z, et al. Synthesis of flower-like MoS2/g-C3N4 nanosheet heterojunctions with enhanced photocatalytic reduction activity of uranium(VI). Appl Surf Sci 2020; 520: 146352. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Zhang Z, Zhou R, Dong Z, et al. Visible-light induced photocatalytic removal of U(VI) from aqueous solution by MoS2/g-C3N4 nanocomposites. J Radioanal Nucl Chem 2021; 328: 9-17. [Article] [Google Scholar]
- Wang T, Zhang ZB, Dong Z, et al. A facile synthesis of g-C3N4/WS2 heterojunctions with enhanced photocatalytic reduction activity of U(VI). J Radioanal Nucl Chem 2022; 331: 577-586. [Article] [Google Scholar]
- Li Z, Zhang Z, Dong Z, et al. Synthesis of MoS2/P-g-C3N4 nanocomposites with enhanced visible-light photocatalytic activity for the removal of uranium (VI). J Solid State Chem 2021; 302: 122305. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Li S, Yang X, Cui Z, et al. Efficient photoreduction strategy for uranium immobilization based on graphite carbon nitride/perovskite oxide heterojunction nanocomposites. Appl Catal B-Environ 2021; 298: 120625. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Li S, Wang Y, Wang J, et al. Modifying g-C3N4 with oxidized Ti3C2 MXene for boosting photocatalytic U(VI) reduction performance. J Mol Liq 2022; 346: 117937. [Article] [Google Scholar]
- Huang X, Xiao J, Mei P, et al. The synthesis of Z-scheme MoS2/g-C3N4 heterojunction for enhanced visible-light-driven photoreduction of uranium. Catal Lett 2022; 152: 1981–1989 [Google Scholar]
- Chen T, Li M, Zhou L, et al. Harmonizing the energy band between adsorbent and semiconductor enables efficient uranium extraction. Chem Eng J 2021; 420: 127645. [Article] [Google Scholar]
- Liu S, Wang Z, Lu Y, et al. Sunlight-induced uranium extraction with triazine-based carbon nitride as both photocatalyst and adsorbent. Appl Catal B-Environ 2021; 282: 119523. [Article] [Google Scholar]
- Gao H, Xu J, Zhou J, et al. Metal organic framework derived heteroatoms and cyano (–C≡N) group co-decorated porous g-C3N4 nanosheets for improved photocatalytic H2 evolution and uranium(VI) reduction. J Colloid Interface Sci 2020; 570: 125-134. [Article] [Google Scholar]
- Li P, Wang Y, Wang J, et al. Carboxyl groups on g-C3N4 for boosting the photocatalytic U(VI) reduction in the presence of carbonates. Chem Eng J 2021; 414: 128810. [Article] [Google Scholar]
- Feng J, Yang Z, He S, et al. Photocatalytic reduction of uranium(VI) under visible light with Sn-doped In2S3 microspheres. Chemosphere 2018; 212: 114-123. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Zhang Z, Li Z, Dong Z, et al. Synergy of photocatalytic reduction and adsorption for boosting uranium removal with PMo12/UiO-66 heterojunction. Chin Chem Lett 2022; 33: 3577-3580. [Article] [CrossRef] [Google Scholar]
- Gu S, Wu S, Cao L, et al. Tunable redox chemistry and stability of radical intermediates in 2D covalent organic frameworks for high performance sodium ion batteries. J Am Chem Soc 2019; 141: 9623-9628. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Xu H, Tao S, Jiang D. Proton conduction in crystalline and porous covalent organic frameworks. Nat Mater 2016; 15: 722-726. [Article] [NASA ADS] [CrossRef] [MathSciNet] [Google Scholar]
- Kuhn P, Antonietti M, Thomas A. Porous, covalent triazine‐based frameworks prepared by ionothermal synthesis. Angew Chem Int Ed 2008; 47: 3450–3453 [Google Scholar]
- Wang K, Yang L, Wang X, et al. Covalent triazine frameworks via a low‐temperature polycondensation approach. Angew Chem Int Ed 2017; 56: 14149-14153. [Article] [Google Scholar]
- Guan X, Li H, Ma Y, et al. Chemically stable polyarylether-based covalent organic frameworks. Nat Chem 2019; 11: 587-594. [Article] [CrossRef] [PubMed] [Google Scholar]
- Liang R, Luo J, Lin S, et al. Boosting the photoreduction uranium activity for donor–acceptor–acceptor type conjugated microporous polymers by statistical copolymerization. Sep Purif Technol 2023; 312: 123291. [Article] [Google Scholar]
- Xu Y, Liu Q, Zhu J, et al. Self-assembled porous polydopamine microspheres modified polyacrylonitrile fiber for synergistically enhanced U(VI) extraction and seawater desalination. Sep Purif Technol 2023; 306: 122684. [Article] [Google Scholar]
- Cui WR, Zhang CR, Liang RP, et al. Covalent organic framework sponges for efficient solar desalination and selective uranium recovery. ACS Appl Mater Interfaces 2021; 13: 31561-31568. [Article] [Google Scholar]
- Li H, He N, Cheng C, et al. Antimicrobial polymer contained adsorbent: A promising candidate with remarkable anti-biofouling ability and durability for enhanced uranium extraction from seawater. Chem Eng J 2020; 388: 124273. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Zhang CR, Cui WR, Niu CP, et al. rGO-based covalent organic framework hydrogel for synergistically enhance uranium capture capacity through photothermal desalination. Chem Eng J 2022; 428: 131178. [Article] [CrossRef] [Google Scholar]
- Zhang F, Dong H, Li Y, et al. In situ metal‐oxygen‐hydrogen modified B-TiO2@Co2P-X S-scheme heterojunction effectively enhanced charge separation for photo‐assisted uranium reduction. Adv Sci 2024; 11: 2305439. [Article] [Google Scholar]
- Li Z, Zhang Z, Dong Z, et al. Solar light-responsive CdS/UiO-66-NH2 for ultrafast uranium reduction from uranium-containing mine wastewater without external sacrificial agents. Sep Purif Technol 2022; 283: 120195. [Article] [Google Scholar]
- Dong Z, Hu S, Li Z, et al. Biomimetic photocatalytic system designed by spatially separated cocatalysts on Z-scheme heterojunction with identified charge‐transfer processes for boosting removal of U(VI). Small 2023; 19: e2300003. [Article] [CrossRef] [Google Scholar]
- Segura JL, Royuela S, Mar Ramos M. Post-synthetic modification of covalent organic frameworks. Chem Soc Rev 2019; 48: 3903-3945. [Article] [Google Scholar]
- Albacete P, Martínez JI, Li X, et al. Layer-stacking-driven fluorescence in a two-dimensional imine-linked covalent organic framework. J Am Chem Soc 2018; 140: 12922-12929. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Miao Z, Liu G, Cui Y, et al. A novel strategy for the construction of covalent organic frameworks from nonporous covalent organic polymers. Angew Chem Int Ed 2019; 131: 4960-4964. [Article] [Google Scholar]
- Wang J, Wang Y, Wang W, et al. Tunable mesoporous g-C3N4 nanosheets as a metal-free catalyst for enhanced visible-light-driven photocatalytic reduction of U(VI). Chem Eng J 2020; 383: 123193. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Jiang P, Yu K, Yuan H, et al. Encapsulating Ag nanoparticles into ZIF-8 as an efficient strategy to boost uranium photoreduction without sacrificial agents. J Mater Chem A 2021; 9: 9809-9814. [Article] [Google Scholar]
- Ye Y, Jin J, Chen F, et al. Removal and recovery of aqueous U(VI) by heterogeneous photocatalysis: Progress and challenges. Chem Eng J 2022; 450: 138317. [Article] [Google Scholar]
- Chen T, Yu K, Dong C, et al. Advanced photocatalysts for uranium extraction: Elaborate design and future perspectives. Coord Chem Rev 2022; 467: 214615. [Article] [CrossRef] [Google Scholar]
- Zhu W, Li X, Wang D, et al. Advanced photocatalytic uranium extraction strategies: Progress, challenges, and prospects. Nanomaterials 2023; 13: 2005. [Article] [CrossRef] [PubMed] [Google Scholar]
- Lu C, Chen R, Wu X, et al. Boron doped g-C3N4 with enhanced photocatalytic UO22+ reduction performance. Appl Surf Sci 2016; 360: 1016-1022. [Article] [Google Scholar]
- Wen C, Yao Y, Meng L, et al. Photocatalytic and electrocatalytic extraction of uranium by COFs: A review. Ind Eng Chem Res 2023; 62: 18230-18250. [Article] [Google Scholar]
- Hong J, Ma R, Wu Y, et al. Experimental and theoretical identifications of durable Fe–Nx configurations embedded in graphitic carbon nitride for uranium photoreduction. J Environ Chem Eng 2022; 10: 108374. [Article] [Google Scholar]
- Wu X, Jiang S, Song S, et al. Constructing effective photocatalytic purification system with P-introduced g-C3N4 for elimination of UO22+. Appl Surf Sci 2018; 430: 371-379. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Lu C, Zhang P, Jiang S, et al. Photocatalytic reduction elimination of UO22+ pollutant under visible light with metal-free sulfur doped g-C3N4 photocatalyst. Appl Catal B-Environ 2017; 200: 378-385. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Li P, Wang J, Wang Y, et al. An overview and recent progress in the heterogeneous photocatalytic reduction of U(VI). J PhotoChem PhotoBiol C-PhotoChem Rev 2019; 41: 100320. [Article] [CrossRef] [Google Scholar]
- Jiang XH, Xing QJ, Luo XB, et al. Simultaneous photoreduction of uranium(VI) and photooxidation of arsenic(III) in aqueous solution over g-C3N4/TiO2 heterostructured catalysts under simulated sunlight irradiation. Appl Catal B-Environ 2018; 228: 29-38. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- He F, Xiao Q, Chen Y, et al. Synergistic reduction of U(VI) and selective oxidation of benzyl alcohol to prepare benzaldehyde via WOx/g-C3N4. Appl Catal B-Environ 2024; 343: 123525. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Wu F, Zhang Z, Cheng Z, et al. The enhanced photocatalytic reduction of uranium(VI) by ZnS@g-C3N4 heterojunctions under sunlight. J Radioanal Nucl Chem 2021; 329: 1125-1133. [Article] [Google Scholar]
- Hu E, Chen Q, Gao Q, et al. Cyano‐functionalized graphitic carbon nitride with adsorption and photoreduction isosite achieving efficient uranium extraction from seawater. Adv Funct Mater 2024; 34: 2312215 [CrossRef] [Google Scholar]
- Li Z, Zhang Z, Zhu X, et al. Exciton dissociation and transfer behavior and surface reaction mechanism in donor–acceptor organic semiconductor photocatalytic separation of uranium. Appl Catal B-Environ 2023; 332: 122751. [Article] [Google Scholar]
- Gong J, Xie Z, Xiong C, et al. Efficient photocatalytic removal of U(VI) over π-electron-incorporated g-C3N4 under visible light irradiation. J Radioanal Nucl Chem 2019; 322: 1115-1125. [Article] [CrossRef] [MathSciNet] [Google Scholar]
- Gong J, Xie Z, Wang B, et al. Fabrication of g-C3N4-based conjugated copolymers for efficient photocatalytic reduction of U(VI). J Environ Chem Eng 2021; 9: 104638. [Article] [Google Scholar]
- Dai Z, Sun Y, Zhang H, et al. Photocatalytic reduction of U(VI) in wastewater by mGO/g-C3N4 nanocomposite under visible LED light irradiation. Chemosphere 2020; 254: 126671. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Molina A, Patil N, Ventosa E, et al. Electrode engineering of redox-active conjugated microporous polymers for ultra-high areal capacity organic batteries. ACS Energy Lett 2020; 5: 2945-2953. [Article] [Google Scholar]
- Wang H, Li Q, Wu Q, et al. Conjugated microporous polymers with bipolar and double redox‐active centers for high‐performance dual‐ion, organic symmetric battery. Adv Energy Mater 2021; 11: 2100381. [Article] [CrossRef] [Google Scholar]
- Yang X, Duan L, Ran X, et al. Conjugated microporous polymer bearing 1,3,4-oxadiazole and thienyl moieties for decomposition of organic dyes under visible light. React Funct Polym 2021; 168: 105051. [Article] [Google Scholar]
- Zhi Y, Ma S, Xia H, et al. Construction of donor-acceptor type conjugated microporous polymers: A fascinating strategy for the development of efficient heterogeneous photocatalysts in organic synthesis. Appl Catal B-Environ 2019; 244: 36-44. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Wang Z, Yang X, Yang T, et al. Dibenzothiophene dioxide based conjugated microporous polymers for visible-light-driven hydrogen production. ACS Catal 2018; 8: 8590-8596. [Article] [Google Scholar]
- Chen L, Chen B, Kang J, et al. The synthesis of a novel conjugated microporous polymer and application on photocatalytic removal of uranium(Ⅵ) from wastewater under visible light. Chem Eng J 2022; 431: 133222. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Chen B, Zhang G, Chen L, et al. Visible light driven photocatalytic removal of uranium(VI) in strongly acidic solution. J Hazard Mater 2022; 426: 127851. [Article] [Google Scholar]
- Yu F, Zhu Z, Li C, et al. A redox-active perylene-anthraquinone donor-acceptor conjugated microporous polymer with an unusual electron delocalization channel for photocatalytic reduction of uranium(VI) in strongly acidic solution. Appl Catal B-Environ 2022; 314: 121467. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Zhang W, Wang B, Cui H, et al. Unveiling the exciton dissociation dynamics steered by built-in electric fields in conjugated microporous polymers for photoreduction of uranium (VI) from seawater. J Colloid Interface Sci 2024; 662: 377-390. [Article] [Google Scholar]
- Yu F, Zhu Z, Wang S, et al. Novel donor-acceptor-acceptor ternary conjugated microporous polymers with boosting forward charge separation and suppressing backward charge recombination for photocatalytic reduction of uranium (VI). Appl Catal B-Environ 2022; 301: 120819. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Chen YR, Wang X, Fan XY, et al. Facilitating charge separation and migration of conjugated microporous polymers via skeleton isomerism engineering for photocatalytic reduction of uranium (VI). Sep Purif Technol 2024; 334: 126121. [Article] [Google Scholar]
- Zhong X, Ren Z, Ling Q, et al. Adsorption-photocatalysis processes: The performance and mechanism of a bifunctional covalent organic framework for removing uranium ions from water. Appl Surf Sci 2022; 597: 153621. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Song Y, Li A, Li P, et al. Unassisted uranyl photoreduction and separation in a donor–acceptor covalent organic framework. Sep Purif Technol 2022; 34: 2771–2778 [Google Scholar]
- Zhao Y, Li S, Fu G, et al. Construction of layer-blocked covalent organic framework heterogenous films via surface-initiated polycondensations with strongly enhanced photocatalytic properties. ACS Cent Sci 2024; 10: 775–781 [Google Scholar]
- Cui W-R, Zhang C-R, Xu R-H, et al. High-efficiency photo-enhanced extraction of uranium from natural seawater by olefin-linked covalent organic frameworks. ACS ES T Water 2020; 1: 440–448 [Google Scholar]
- Cui W, Zhang C, Xu R, et al. Low band gap benzoxazole‐linked covalent organic frameworks for photo‐enhanced targeted uranium recovery. Small 2021; 17: e2006882. [Article] [CrossRef] [PubMed] [Google Scholar]
- Wu Y, Li P, Chen L, et al. Reversible amine-to-imine chemistry at a covalent organic framework for sustainable uranium redox separation. ACS Sustain Chem Eng 2024; 12: 6659-6665. [Article] [Google Scholar]
- Zhong X, Ling Q, Kuang P, et al. Modified side-chain COFs construct built-in electric fields with low exciton binding energy for photo-reduced uranium. Chem Eng J 2024; 483: 149339. [Article] [Google Scholar]
- Zhang S, Chen L, Qu Z, et al. Confining Ti-oxo clusters in covalent organic framework micropores for photocatalytic reduction of the dominant uranium species in seawater. Chem 2023; 9: 3172-3184. [Article] [Google Scholar]
- Chen L, Hang J, Chen B, et al. Photocatalytic uranium removal from basic effluent by porphyrin-Ni COF as the photocatalyst. Chem Eng J 2023; 454: 140378. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Liu JL, Lin MX, Huang J, et al. sp2-c linked Cu-based metal-covalent organic framework for chemical and photocatalysis synergistic reduction of uranium. Chem Eng J 2024; 491: 151982. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Cui WR, Li FF, Xu RH, et al. Regenerable covalent organic frameworks for photo-enhanced uranium adsorption from seawater. Angew Chem Int Ed 2020; 132: 17837-17843. [Article] [Google Scholar]
- Hao M, Chen Z, Liu X, et al. Converging cooperative functions into the nanospace of covalent organic frameworks for efficient uranium extraction from seawater. CCS Chem 2022; 4: 2294-2307. [Article] [CrossRef] [Google Scholar]
- Yu Q, Yuan Y, Wen J, et al. A universally applicable strategy for construction of anti‐biofouling adsorbents for enhanced uranium recovery from seawater. Adv Sci 2019; 6: 1900002. [Article] [CrossRef] [Google Scholar]
- Hao M, Xie Y, Liu X, et al. Modulating uranium extraction performance of multivariate covalent organic frameworks through donor–acceptor linkers and amidoxime nanotraps. JACS Au 2023; 3: 239-251. [Article] [CrossRef] [PubMed] [Google Scholar]
- Zhang HL, Liu W, Li A, et al. Three mechanisms in one material: Uranium capture by a polyoxometalate-organic framework through combined complexation, chemical reduction, and photocatalytic reduction. Angew Chem Int Ed 2019; 58: 16110–16114 [Google Scholar]
- Chen M, Liu T, Tang S, et al. Mixed-linker strategy toward enhanced photoreduction-assisted uranium recovery from wastewater and seawater. Chem Eng J 2022; 446: 137264. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Li H, Zhai F, Gui D, et al. Powerful uranium extraction strategy with combined ligand complexation and photocatalytic reduction by postsynthetically modified photoactive metal-organic frameworks. Appl Catal B-Environ 2019; 254: 47-54. [Article] [Google Scholar]
- Zhao J, Lyu C, Zhang R, et al. Self-cleaning and regenerable nano zero-valent iron modified PCN-224 heterojunction for photo-enhanced radioactive waste reduction. J Hazard Mater 2023; 442: 130018. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Chen M, Liu T, Zhang X, et al. Photoinduced enhancement of uranium extraction from seawater by MOF/black phosphorus quantum dots heterojunction anchored on cellulose nanofiber aerogel. Adv Funct Mater 2021; 31: 2100106. [Article] [CrossRef] [Google Scholar]
- Zhong X, Liu Y, Zeng W, et al. Excellent photoreduction performance of U(VI) on metal organic framework/covalent organic framework heterojunction by solar-driven. Sep Purif Technol 2022; 285: 120405. [Article] [CrossRef] [Google Scholar]
- Bi RX, Liu X, Lei L, et al. Core-shell MOF@COF photocatalysts for synergistic enhanced U(VI) and tetracycline cleanup through space and carrier separation. Chem Eng J 2024; 485: 150026. [Article] [CrossRef] [Google Scholar]
- Yang Y, Guo K, Zhu M, et al. Exploring electron transfer mechanism in synergistic interactional reduced polyoxometalate-based Cu(I)–organic framework for photocatalytic removal of U(VI). Inorg Chem 2024; 63: 7876-7885. [Article] [CrossRef] [PubMed] [Google Scholar]
- Liu T, Tang S, Wei T, et al. Defect-engineered metal-organic framework with enhanced photoreduction activity toward uranium extraction from seawater. Cell Rep Phys Sci 2022; 3: 100892. [Article] [Google Scholar]
- Gao Z, Wang Y, Lin Y, et al. Constructing dual-functional porphyrin-based thorium metal-organic framework toward photocatalytic uranium(VI) reduction integrated with organic oxidation. Sci China Chem 2022; 65: 1544-1551. [Article] [CrossRef] [Google Scholar]
- Yu Z, Ye D, Zhao J, et al. Photocatalytic anti-biofouling coatings with dynamic surfaces of hybrid metal-organic framework nanofibrous mats for uranium (VI) separation from seawater. Chem Eng J 2021; 420: 129691. [Article] [Google Scholar]
- Salomone VN, Meichtry JM, Zampieri G, et al. New insights in the heterogeneous photocatalytic removal of U(VI) in aqueous solution in the presence of 2-propanol. Chem Eng J 2015; 261: 27-35. [Article] [Google Scholar]
- Pan Z, Bártová B, LaGrange T, et al. Nanoscale mechanism of UO2 formation through uranium reduction by magnetite. Nat Commun 2020; 11: 4001. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Wang Z, Liu H, Lei Z, et al. Graphene aerogel for photocatalysis-assist uranium elimination under visible light and air atmosphere. Chem Eng J 2020; 402: 126256. [Article] [Google Scholar]
- Dong Z, Zhang Z, Li Z, et al. Double-shelled hollow nanosphere assembled by TiO2@surface sulfate functionalized CdS for boosting photocatalysis reduction of U(VI) under seawater conditions. Chem Eng J 2022; 431: 133256. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Pei H, Dong Z, Li Z, et al. Synergistic effect of homojunction and Ohmic junctions in CdS boosting spatial charge separation for U(VI) photoreduction. Nano Res 2024; 17: 6849-6859. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Gong X, Tang L, Wang R, et al. Achieving efficient photocatalytic uranium extraction within a record short period of 3 min by up-conversion erbium doped ZnO nanosheets. Chem Eng J 2022; 450: 138044. [Article] [Google Scholar]
All Tables
All Figures
 |
Figure 1 Possible mechanism for photocatalytic reduction of uranium by polymers. |
| In the text | |
 |
Figure 2 (a) Manufacturing process, UV-Vis DRS spectra and bandgaps of conjugated microporous polymers [25]. (b) Estimated band structures of PCN and KCN-PHI [27]. (c) Estimated band structures of CCN and D-CCN [28]. (d) Schematic illustration and DRS spectra of the TTA-UC ternary nanohybrid preparation [31]. (e) Estimated band structures of Co3O4@TiO2@CdS@Au [32]. (f) Calculated spatial distributions of LUMO and HOMO in the Tp-TMT, Hb-TMT and Tb-TMT [35]. |
| In the text | |
 |
Figure 3 (a) The PL and TR-PL of the six CN-modified N-CMPs [40]. Design and synthesis of the (b) PCTF-1 [44], (c) Nx–COFs [45], (d) sp2c-COF and sp2c-COFERDN [47]. (e) Schematic illustration of four types of heterojunctions. |
| In the text | |
 |
Figure 4 (a) Modification strategy of g-C3N4. (b) Molecular orbital energies and structures of the g-C3N4 and S-g-C3N4 as well as the coordination complexes with U(VI) ion [91]. (c) Schematic diagram of the mechanism of g-C3N4/TiO2 photocatalytic reduction of U(VI) and oxidation of As(III). (d) Photoreduction of U(VI) and the oxidation of As(III) by g-C3N4/TiO2 [94]. (e) Schematic diagram of g-C3N4-CN agar aerogel seawater uranium extraction device. (f) Performance of g-C3N4-CN agar aerogel for uranium extraction from seawater [97]. (g) Mesoporous g-C3N4 photocatalytic U(VI) extraction from seawater. (h) Extractability of mesoporous g-C3N4 for uranium in deionized water and seawater. (i) The U LIII-edge XANES spectra of U(VI)-loaded mesoporous g-C3N4 after irradiation [99]. |
| In the text | |
 |
Figure 5 (a) The photoreduction mechanism of U(VI) over COFs skeleton , (b) LB-COF [115], and (c) Tp-DBD COF [116]. (d) Photochemical reduction and recycling experiment of PI-3-AR [117]. (e) DFT on diverse adsorption configurations and energetics for UO22+ binding to TpBD-X (001) and the elucidated rendering delineates the photocatalytically promoted U(VI) reaction mechanism [119]. |
| In the text | |
 |
Figure 6 (a) The synthesis method and mechanism of U(VI) photoreduction in SnS2COF [36], and (b) BiOBr@TpPa-1 [51]. (c) Estimated energy levels of UO2(CO3)34−, COF-TZ, TiOCs, TiOCs⊂COF-TZ, and UO2(CO3)34−@TiOCs⊂COF-TZ [120]. (d) Mechanism of H2O2 photosynthesis and uranyl removal in TT-Por COF-Ni [121]. (e) Graphic view of the eclipsed AA stacking structure of Cu-TMT [118]. (f) The band structure diagram of Cu3(PyCA)3 and Cu-TMT [122]. |
| In the text | |
 |
Figure 7 (a) The synthesis of π-conjugated NDA-TN-AO and uranium extraction capacity [124]. (b) Synthetic scheme of COF 4 and COF 4-Pd-AO and U LIII-edge XANES spectra for COF 4-Pd-AO after uranium extraction studies [125]. (c) Uranyl adsorption kinetics on COF 2-Ru-AO at an initial uranium concentration of ~9 ppm, ~27 and 270 ppb in uranyl-spiked seawater, respectively. (d) Estimated energy levels of COF 2-Ru-AO [126]. |
| In the text | |
 |
Figure 8 (a) Schematic illustration of the proposed mechanism on photocatalytic reduction of U(VI) for TCPP(Ni)⊂MOF-3 [128], and (b) NZVI@PCN-224 [130]. (c) Stability and antibacterial activity of BP@CNF-MOF after exposure to natural seawater for 0, 15, and 30 days [133]. (d) Proposed mechanism on photocatalytic reduction of U(VI) for Ti-MOF@DATp [134], (e) POMOF and (f) Cu@Th-TCPP [136]. (g) SEM images (scale bar, 5 μm) of the AOP@ZIF-8/TA fibers after each elution [137]. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

