| Issue |
Natl Sci Open
Volume 4, Number 2, 2025
Special Topic: Flexible Electronics and Micro/Nanomanufacturing
|
|
|---|---|---|
| Article Number | 20230080 | |
| Number of page(s) | 22 | |
| Section | Engineering | |
| DOI | https://doi.org/10.1360/nso/20230080 | |
| Published online | 05 March 2024 | |
REVIEW
Recent progress on acoustofluidic manipulation of cells and particles
1
Frontier Institute of Science and Technology (FIST), Xi’an Jiaotong University, Xi’an 710049, China
2
Pratt School of Engineering, Duke University, Durham, NC 27705, USA
3
School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138, USA
4
Micro- and Nano-technology Research Center, State Key Laboratory for Manufacturing Systems Engineering, Xi’an Jiaotong University, Xi’an 710049, China
* Corresponding author (email: This email address is being protected from spambots. You need JavaScript enabled to view it.
)
Received:
1
December
2023
Revised:
11
January
2024
Accepted:
29
February
2024
Abstract
Acoustofluidics, an interdisciplinary nexus of microfluidics and acoustics, is propelling the critical functionalities of manipulation, separation, and mixing within microscale environments. This integration leverages the accuracy of microfluidics with the manipulation capabilities of acoustics, thereby enhancing the vital sample processing steps and satisfying inquiries in experiments. To fulfill the requisites of practical application in clinical and research arenas, the evolution of acoustofluidics concentrates on accomplishing finer particle separation, instantaneous manipulation, and augmented integration capacity. Acoustofluidics has evolved into a sophisticated and versatile instrument for handling specimens and reactants, prompting a trend towards devices characterized by stable performance at elevated frequencies, programmable control, and seamless integration with auxiliary microfluidic systems. In this review, we present the latest advancements in the development of sophisticated acoustofluidic systems that enhance efficiency and enable precise modulation of performance across spatial and temporal scales, thereby extending their functionality and suitability for practical applications.
Key words: acoustofluidics / microfabrication / acoustics / microfluidics
© The Author(s) 2024. Published by Science Press and EDP Sciences.
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Microfluidic-based manipulation systems are rapidly advancing, showing significant promise for medical applications. The miniaturization characteristic of these technologies is pivotal, enabling advanced capabilities in medical diagnostics and synthesis by facilitating processes such as mixing and separation [1,2], which are fundamental operations in clinical settings. Mixing enables essential molecular interactions for reactions in diagnostic assays and synthetic processes [3,4], while separation is indispensable for purification [5], targeted molecule isolation [6], and cell sorting [7]. Achieved through precise fluid or constituent manipulation, these processes benefit from the reduced scales of microfluidic environments. Unlike centrifugation or bulk mixing, which require centimeter-scale cell travel or diffusion over microliter volumes, microfluidic systems operate at the micro- to sub-micrometer scale, significantly enhancing mixing and separation efficiency.
Microfluidic systems are classified by their manipulation mechanisms into passive and active modalities. Passive systems use intrinsic channel geometries to facilitate operations like filtration and rapid separation, where fluid flow interacts with microscale structures without the need for external inputs beyond active pumping units [8,9]. Passive microfluidic systems typically utilize mechanisms such as inertial flow, deterministic lateral displacement (DLD), hydrodynamic fractionation, and Dean flow fractionation [10,11]. These methods exploit complex flow fields for precise manipulation functions, requiring a finely tuned interplay between fluid dynamics and the geometrical structures of the microfluidic channels. Specifically, the inertial flow approach necessitates increased flow rates, adjusted according to channel size and fluid viscosity, to transition from laminar flow to Stokes flow or turbulence [12]. DLD is achieved through the lateral displacement of particles, dictated by their interactions with strategically placed obstacles within the channel [13]. Hydrodynamic fractionation hinges on shear-induced migration, controlled by carefully calibrated flow rates and fluid viscosity [14]. Finally, Dean flow fractionation is based on the relationship between flow rate and channel curvature, inducing secondary rotational flows crucial for effective manipulation [15]. These systems, which utilize structural elements to induce complex flow dynamics, are less versatile than active approaches due to the need for consistent conditions regarding flow rates, channel design, and particle characteristics [16,17]. In contrast, active microfluidic systems employ external forces such as optical [18], electrical [19], magnetic [20], or acoustic energy [21], to directly manipulate particles. Although these systems require greater energy inputs and intricate manufacturing processes, they provide enhanced adaptability. This is because the intensity of the external forces can be modulated through power adjustments, and the targets of these forces can be selectively determined through various methods, including programmable controls and integration with detection units.
Acoustofluidic systems, in particular, utilize acoustic waves to manipulate specific microfluidic system components, offering a non-contact method for active manipulation [22]. A typical device consists of a microfluidic channel and a transducer to generate an acoustic field. Manipulation occurs either through drag forces from acoustic streaming or directly from acoustic radiation forces due to wave scattering on particle surfaces. The frequency of the acoustic waves, which ranges from kilohertz to megahertz, determines the device’s functionality and manipulation specificity [23,24]. Employed at frequencies that cause low cellular and tissue damage, these devices are highly biocompatible and capable of handling entities from micrometer-sized embryos [25,26], to nanometer-sized microvesicles [27,28]. Acoustofluidic systems have excelled in applications such as white blood cell apheresis [29], circulating tumor cell retrieval [30], and exosome isolation [31], showing substantial potential for enhancing point-of-care and early diagnostic applications.
Efforts are currently underway to transition acoustofluidic systems from experimental setups to practical applications. The technology is renowned for enabling high-resolution separation on compact platforms [32,33], beneficial for diagnostic and research platforms as isolation and control units. Real-world applications, however, demand robust stability and real-time adjustability for dynamic control. To meet these demands, advancements focus on reducing interference between acoustic radiation forces and streaming [34], enhancing manipulation flexibility [35], and integrating with more functional microfluidic systems [36]. These developments not only increase the systems’ complexity but also broaden their potential applications, but addressing them presents numerous challenges in the field of acoustofluidics. These challenges primarily arise due to the coexistence of the two fundamental effects: acoustic radiation force and acoustic streaming [23,37]. Despite their similar mechanisms of generation, these effects can produce conflicting outcomes [38,39]. In earlier conventional devices, the influence of the non-dominant effect was often negligible for applications. However, as demands have shifted towards handling smaller samples and broader manipulation capabilities, increased device power or frequency have made the non-dominant effect significantly more impactful. To enhance manipulation outcomes in acoustofluidics, a deep understanding of the roles and interactions between acoustic radiation force and acoustic streaming is essential, particularly in terms of target specificity and interference patterns. To provide suggestions for minimizing interference and harnessing the synergistic potential of these acoustic effects, which are crucial for the future development of acoustofluidic technology, this review delves into recent technological advancements. It highlights the strategic use of acoustic radiation force and acoustic streaming for precise target manipulation and the mitigation of interferences. By offering a comprehensive overview of these advancements, this analysis aims to inform and guide the development of more effective acoustofluidic designs, tailored to meet the intricate requirements and challenges of contemporary applications.
THEORY AND MECHANISM
A typical acoustofluidic device contains a microfluidic unit for sample processing and an acoustic unit for generating either surface acoustic wave (SAW) or bulk acoustic wave (BAW) for generating acoustic field in the channel for manipulation purpose (Figure 1A). Acoustic manipulation is primarily governed by the acoustic radiation force and acoustic streaming, which are noted as the primary and secondary forces, depending on whether the force is directly derived from the acoustic wave or induced by acoustically generated streaming. The predominance and resultant effects of these mechanisms vary with factors such as wave type, frequency, amplitude, and particle size [39,40]. Acoustic radiation force arises from an imbalance in acoustic pressure on the surface of particles, attributed to the scattering effect of the particles on the propagating acoustic waves (Figure 1B) [39]. Conversely, acoustic streaming encompasses a broader range of fluid movement induced by acoustic vibrations; this includes microstreaming from microbubbles [41] or sharp edges [42] activated by acoustic waves. However, for clarity in this review, we will limit our discussion of acoustic streaming to instances where fluid movement is directly instigated by acoustic wave-driven compression rather than by the vibration of intermediary structures. In the context of particles suspended in a fluid, the acoustic radiation force can be regarded as acting directly upon them, while acoustic streaming moves particles by first generating a flow (Figure 1C) [43]. Although these phenomena propel particles via distinct pathways, they are theoretically underpinned by similar principles, namely the Navier-Stokes equations [44].
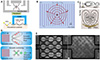 |
Figure 1 Mechanisms of acoustic manipulation include acoustic radiation force and acoustics streaming. (A) Basic design of acoustofluidic devices, the device using bulk acoustic wave (upside schematics) featured by acoustic wave propagating in the bulk of the liquid, while surface acoustic wave device (down side schematics) featured by the Rayleigh wave propagating in the surface of the substrate [22]. (B) The mechanism of acoustic radiation force is the scattering of acoustic wave on the particle’s surface, indicating that the particle is directly manipulated by the acoustic field [39]. (C) The generation of acoustic streaming derives from the compression of fluid within the acoustic field; particle manipulation is achieved by generated streaming [43]. (D) Schematics of the dominance of acoustic radiation force and acoustic streaming in particle manipulation, a same acoustofludic device generates both effects, normally smaller particles are manipulated by acoustic streaming effect (up), while larger particles are manipulated by acoustic radiation force [53]. (E) Practical analysis of in a same acoustic field, larger particles are dominant by acoustic radiation force (left) and smaller particles follows the pattern of acoustic streaming (right) [54]. |
Since an acoustic wave induces harmonic particle vibrations in the medium, it naturally leads to fluid compression, thereby causing fluctuations in density and pressure. These fluctuations can be described by the Navier-Stokes equations as
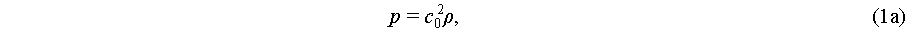 (1a)
(1a)
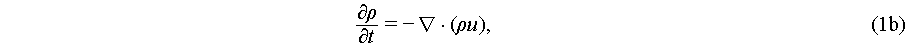 (1b)
(1b)
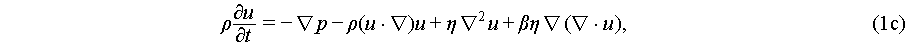 (1c)
(1c)
where p, ρ, and c0 are the pressure, density, and speed of sound in the fluid, respectively. Fluid viscosity η and viscosity ratio β are introduced for predicting streaming velocity u of a linear viscous fluid. Both acoustic radiation force and acoustic streaming are characterized by the fluid’s velocity, density, and pressure. However, the mathematical characterization of these fluidic parameters within an acoustic field is an ongoing area of research, with current models primarily based on slow streaming representations within an asymptotic expansion framework [45]:
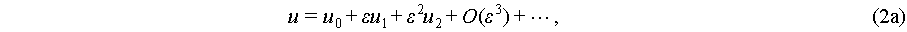 (2a)
(2a)
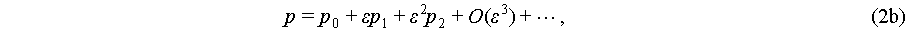 (2b)
(2b)
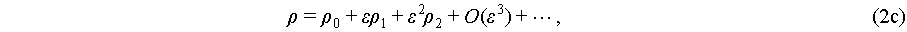 (2c)
(2c)
where the parameter ε demonstrates order differentiation. The first orders in these equations express the fluctuations of velocity, pressure, and density governed by harmonic oscillations brought by acoustic wave. Due to the ideal time average value of a harmonic oscillation is zero, the first orders become periodic components [45], while the expressions of pressure and velocity are based on the expanding of the second orders as the steady components. For the expression of acoustic pressure, the second orders of Eq. (2) could be expressed by a one-step second order approximation, which could be simplified as ε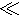 1 [38]. The expression of acoustic streaming is more complicated with a successive first to second order approximation approach [46].
1 [38]. The expression of acoustic streaming is more complicated with a successive first to second order approximation approach [46].
Acoustic radiation force corresponds with acoustic radiation pressure, which is ex-pressed by the Cauchy theorem as a time-dependent domain within the fluid is considered as R(t) [47]. The bounding surface between fluid and particle is denoted as S(t) [48]. Force applied on the S(t) is expressed as
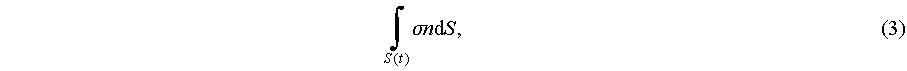 (3)
(3)
where n is a normal of outward unit, and σ is the fluid Cauchy stress. The express of acoustic radiation pressure assumes acoustic wave harmonically excites the fluid, resulting in an oscillation much smaller than acoustics induced streaming in time scale. So Eq. (3) could be used for expressing the time average force during a harmonica excitation [49]:
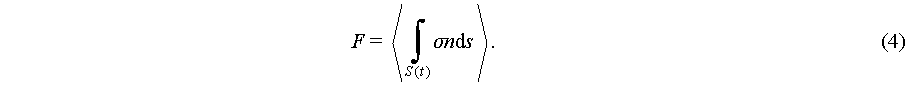 (4)
(4)
To express the approximation second order pressure, the time average acoustic pressure then could be generally expressed as
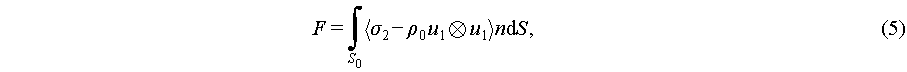 (5)
(5)
where S0 is an unperturbed S(t) configuration, σ2 demonstrates second-order stress, and Reynolds stress, which is the total fluid stress calculated from Navier-Stokes equations, is demonstrated as 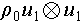 . The mathematical expression of the acoustic radiation force on an inviscid fluid infiltrated spherical particles is [50]
. The mathematical expression of the acoustic radiation force on an inviscid fluid infiltrated spherical particles is [50]
 (6)
(6)
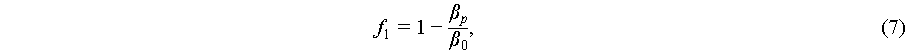 (7)
(7)
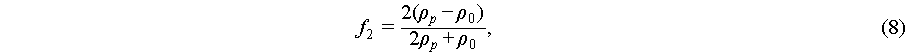 (8)
(8)
where a is the particle radius, βp and β0 are the compressibility of the particle and fluid, and ρp and ρ0 are the density of the particle and fluid, respectively.
The expression of acoustic streaming is based on the conservation of mass and momentum [46]. Using the one-step approximation expressed in Eq. (2), the second order field is negligible, but successive approximation from first order to second order could lead to non-zero value of density, pressure, and velocity in second order [51]. Thus, momentum in a full oscillation could be expressed as the Navier-Stokes equations with second orders [46]:
 (9a)
(9a)
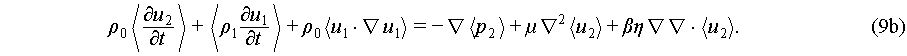 (9b)
(9b)
Values of the velocity u2 of this second order field are non-zero in time-average scale and are so-called acoustic streaming, which perform as a steadily moving fluid by absorbing momentum and energy from the acoustic wave. Once the acoustic streaming is generated, it can manipulate particles through Stokes drag force [52]:
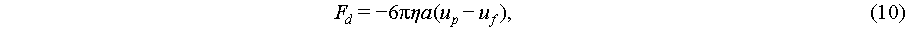 (10)
(10)
where up and uf are the velocity of the particle and fluid.
Theoretical analysis reveals that both acoustic radiation force and acoustic streaming are governed by the Navier-Stokes equations and manifest as second-order, non-zero time-averaged phenomena. A significant distinction arises in the treatment of these second-order effects, wherein the assumptions for acoustic radiation force simplify the approximation by treating the fluid as a uniform density field, whereas acoustic streaming necessitates successive approximations to incorporate density variations from compression. It is important to clarify that the categorization into “primary” and “secondary” forces does not imply a strength comparison between acoustic radiation force and acoustic streaming. Given their similar mechanistic origins, these phenomena inherently coexist and may interact, potentially complicating acoustofluidic manipulation. The dominance of acoustic radiation force is typically influenced by particle size and the density contrast with the surrounding fluid (Figure 1D and E) [53,54]. Conversely, determining the dominance of acoustic streaming involves factors such as the channel’s shape [55], resonance between the channel and the acoustic wave [56], and the wave’s frequency [32]. In a stable-frequency acoustic field, larger particles are generally influenced by acoustic radiation force, whereas smaller particles are subject to acoustic streaming [57]. Nonetheless, acoustofluidic manipulation is advancing towards more precise control, including at the sub-micron level, where the dominant mechanism is not as easily discernible. With the development of simulation techniques, especially efforts including simulating successive two-dimensional models [58], 3D models [59], multi-frequency models [60], and resonator models [61,62], there is a better prediction of acoustic effects on application senses. However, the most fundamental mechanisms of the generation of acoustic radiation force and acoustic streaming are consistent, indicating the inherent coexistence of the two effects cannot be eliminated but only weakened by design. The following section will address recent empirical efforts to optimize the performance of either acoustic radiation force or acoustic streaming in acoustofluidic manipulation.
DEVELOPMENT IN ACOUSIC RADIATION FORCE MANIPULATION
Microfluidic devices that leverage acoustic radiation force exhibit remarkable promise in cell separation, affording precise control, enhanced resolution in particle discrimination, and minimal disruption. Unlike acoustic streaming-based manipulation, which relies on fluid flow to exert drag force, acoustic radiation force enables instantaneous particle manipulation through the direct application of acoustic pressure. The formation speed of an acoustic field, suggested by the condition ε << 1, is significantly faster than that of a streaming field, with the fluid-to-acoustic wave velocity ratio typically below 1/1000. High resolution is another merit of acoustic radiation force, notably in sizing-based particle differentiation. As indicated in Eq. (6), acoustic radiation force is directly proportional to particle volume, whereas Eq. (10) delineates that drag force varies linearly with particle radius. Due to the cubic relationship between volume and radius, slight variations in radius are magnified in terms of volume, resulting in a more pronounced difference in acoustic radiation force in contrast to drag force. Furthermore, minimized disruption is a key advantage, stemming from the deliberate suppression of acoustic streaming. This is particularly pertinent when handling micrometer-sized particles, as devices can be engineered to focus on generating acoustic radiation force, thereby preventing the unintended streaming that could induce mixing in laminar flow, risking sample contamination or dilution [63]. Collectively, these benefits establish acoustic radiation force as a superior method for the manipulation of micrometer-scale particles, providing rapid operation, high size resolution, and high biocompatibility. Such advantages have led to its widespread implementation in various cell manipulation applications, including apheresis [29], the isolation of circulating tumor cells [64], and the differentiation of white and red blood cells [65].
However, the efficacy of acoustic radiation force diminishes when manipulating sub-micrometer and nanometer-sized particles, primarily because the strength of the force declines cubically as particle size decreases. To enhance acoustic radiation force, two strategies have been employed: increasing the acoustic wave frequency and amplifying the voltage applied to the transducer [66]. A higher frequency enhances acoustic pressure fluctuations across a particle’s surface, which is essential for the acoustic radiation force, while an increased voltage strengthens the resultant acoustic field [67]. Though effective, these strategies inadvertently increase acoustic streaming, thus posing a significant challenge. To mitigate the impact of acoustic streaming, the use of standing wave fields has proven to be an effective tactic. In the context of exosome separation, two interdigital transducers (IDTs) generate opposing surface acoustic waves to form a standing wave field within the microfluidic channel (Figure 2A) [28]. This differs from traveling waves, which yield a non-zero time-averaged particle velocity in the second order; standing waves produce equal and opposite velocities in the second order, thereby counteracting the extensive acoustic streaming that typically accompanies traveling waves. Moreover, since particle manipulation by surface acoustic waves (SAWs) is achieved through a leakage wave that travels vertically from the substrate into the fluid, placing a glass slide atop the microfluidic channel enhances the vertical standing wave field (Figure 2B) [68]. As a result, the standing waves are formed both laterally and vertically, causing the acoustic streaming to be confined to regions between the pressure nodes. This advanced arrangement facilitates the isolation of exosomes from complex biological fluids such as blood.
 |
Figure 2 Progress on acoustic radiation force-based subnano- and nanometer size particles manipulation. (A) Exosomes, a subpopulation of 30–200 nm macrovesicles, are separated from blood sample using double separation module with increased frequency SAW fields [28]. (B) The approach for generating lateral and vertical standing wave fields, lateral standing wave field is generated by opposite propagating traveling waves from double IDTs, and vertical standing wave field is generated by the leakage waves and reflected leakage waves from glass bottom of the channel [68]. (C) The high frequency IDT generates uneven acoustic field with the center area dominated by acoustic radiation force and edge areas dominated by acoustic streaming caused vortices, parameters including aperture length of IDT and channel structure can influence this uneven distribution (left), this phenomenon is caused by diffraction during acoustic wave propagation, which induces distribution deterioration with distance increasing [34]. (D) Utilization of unidirectional IDTs can generate stronger acoustic fields with less uneven distribution, indicating improvement on IDT design can avoid diffraction problems [76]. |
While employing standing waves can mitigate acoustic streaming, it is impossible to completely eliminate undesired disruptive flow due to diffraction during acoustic wave propagation. In a parallel IDT composed of multiple alternately arranged finger electrodes, the generated acoustic field contains a central area with uniformly distributed acoustic pressure strength (Figure 2C) [34]. However, the edge areas of the aperture exhibit diffracted and unevenly distributed acoustic pressure. This diffraction-caused uneven distribution of acoustic pressure deteriorates with the propagation of the acoustic wave, resulting in regions far from the IDT where only a small area maintains uniform parallel acoustic pressure, while most areas are dominated by uneven acoustic pressure [69,70]. In an acoustofluidic device, the direct consequence of this diffraction is that only the region parallel to the center of the IDT’s aperture can derive effective acoustic radiation force-based manipulation. Conversely, the peripheral regions parallel to the edges of the aperture are dominated by lateral vortices that can induce mixing and disrupt the intended outcomes of acoustic radiation force manipulation [71]. These undesired lateral vortices cannot be eliminated by the standing wave field since the differences in propagation distance between opposite traveling waves cause variations in acoustic pressure. Factors contributing to diffraction include scattering by the electrodes within the IDT, the ratio of the IDT aperture to the acoustic field wavelength, and the propagation distance [34,72,73]. Each finger electrode acts as a scattering source for the acoustic wave, and high-frequency IDTs, which contain a larger number of finger electrodes than low-frequency IDTs, are more susceptible to diffraction [74,75]. To inhibit diffraction-induced peripheral vortices, one conventional solution is to widen the aperture, thus generating broader areas where the acoustic radiation force is dominant. Decreasing the distance between the IDTs and the microfluidic channel can also help to reduce diffraction by shortening the propagation distance. However, the former solution only increases the manipulation outcome’s tolerance to peripheral vortices, while the latter raises concerns about biocompatibility, as the microfluidic channel is more likely to be affected by heat generated by the IDTs. Recently, efforts have been made to utilize advanced IDT designs derived from electromagnetic research [74]. By carefully adjusting the length of each finger electrode and the gap between electrodes, scattering from the IDT can be significantly suppressed, resulting in a more uniformly distributed acoustic field. The unidirectional IDT has been proven to maintain its feature of generating a surface acoustic wave in one direction on the substrate (Figure 2D) [76]. This characteristic not only facilitates nanoparticle screening but also generates a strong acoustic field suitable for variable microfluidic channels with PDMS bottoms, which have higher impedance to acoustic wave propagation. As a conclusion, since on acoustic radiation force derived nanosized particle manipulation the transducers are necessary for working in high frequency and high power for generated strong pressure fields, the generation of strong non-desired acoustic streaming is inevitable on normal parallel-IDTs. To overcome this obstacle, applying specific design of IDTs with low scattering effects or using theory on acoustic topological insulators, which can achieve stronger directional propagation of acoustic wave, are valuable candidates [77,78]. However, technologies for beam direction control and scattering inhibition IDTs are common in antenna research, which mainly investigate using IDTs for generating electromagnetic wave [79]. The application of these technologies for generating acoustic waves and application of acoustofluidics still require further development. Also, previous acoustic topological insulators are mainly achieved on bulk waves with rather lower frequency, so the integration on high frequency SAW is still a problem.
Compared with the challenges of nanoparticle manipulation, progress in precise, controllable manipulation has yielded attractive achievements. Square pattering of cells and particles could be achieved through stand acoustic fields, but this approach cannot derive flexible manipulation (Figure 3A) [80]. By employing a pair of semicircle IDTs with a gradient in finger spacing, adjusting the frequency of the input current can change the active areas of the IDTs, resulting in an acoustic field with an adjustable direction [81]. This feature has been applied to the manipulation of bulk cells and can achieve the assembly of multiple fibers and nested tissue structures (Figure 3B) [82]. Frequency-adjustable acoustofluidics also facilitates single-cell manipulation. By integrating multiple IDT units with varying finger spacings, an adjustable standing wave field can precisely control the movement of individual cells [83]. This technology has proven effective for cell tweezers, offering the advantage of simultaneously manipulating multiple single cells, separating cell pairs, and quantifying the adhesive forces between cells (Figure 3C) [84]. This flexible manipulation also shows capability of tissue level manipulation with a successful application on controllable neuron organoid fusion [85]. These advances underscore that by harnessing control over frequency, the manipulation dictated by acoustic radiation forces can become programmable, thereby achieving real-time manipulation capabilities. Currently, the focus in the development of acoustic radiation for micrometer-sized particle manipulation is on deriving complex structures and achieving flexible manipulation. On the structure construction aspect, recent progress for using acoustic radiation force for deriving heterotypic cell spheroids [86], vessels [87], and organoids [88–90], indicates the capability of acoustic radiation force for noncontact manipulation. Achieving the forming of more complicated structures with higher heterogeneity, which indicates a targeted manipulation of specific population of particles or cells, would be a research direction for meeting application side requirements. On the flexible manipulation branch, while this approach is less affected by acoustic streaming, it faces unique challenges in integration with continuous microfluidic systems. Existing platforms typically operate in static environments like Petri dishes or square chambers, halting the microfluidic flow during operation. However, continuous flow systems offer greater potential for high-throughput analysis and long-term culturing, both of which are crucial in cellular research. Therefore, exploring the integration of flexible manipulation within a flowing environment represents a promising next step in this field.
 |
Figure 3 Progress on acoustic radiation force derived flexible manipulation. (A) Stable particles patterning with stand acoustic wave field [80]. (B) Circular, slanted-finger IDTs can generated adjustable directional acoustic waves by changing the frequency of excitation currency, change on frequency causes excitation area on the IDT changes, which cause propagation direction change and can achieve flexible manipulation [82]. (C) Harmonic IDTs composed of N individual transducers for harmonic SAWs derives selective and reversible assembly of colloidal crystals or cells by Fourier-synthesized harmonic waves [84]. |
DEVELOPMENT IN ACOUSTIC STREAMING MANIPULATION
Although acoustic streaming-based manipulation cannot achieve the high resolution and rapid response of acoustic radiation force, its inherent features of high strength and extensive range provide different possibilities for manipulation. The high strength of acoustic streaming is built on the independence from the density difference between the particle and fluid. According to Eq. (5), the strength of the acoustic radiation force is low for controlling particles with a density similar to that of the fluid, since changes in medium density lead to acoustic wave scattering, which generates the radiation force. In contrast, acoustic streaming drives manipulation through drag force and can manipulate particles with a density similar to that of the fluid. The wide range advantage indicates that acoustic streaming can move particles over longer distances and cover a larger functional area than acoustic radiation force. In acoustic manipulation within standing wave fields, the particle movement range is limited between pressure nodes; even for traveling waves, the non-zero time-averaged value of pressure is of second order, which is low, causing particles to mainly exhibit harmonic fluctuating motion. On the other hand, due to the continuity of fluid motion, acoustic streaming can produce directional flow that spans multiple pressure nodes, driving continuous particle motion [91,92]. This continuity also allows for acoustic streaming to extend over a longer range or to change direction [93,94], forming circulating flows or vortices [95]. These characteristics provide acoustic streaming with advantages in manipulating large objects, long-distance manipulation, and large scale mixing [96].
The critical parameter for achieving a dominant acoustic streaming effect is the generation of an unbalanced traveling wave acoustic field distribution. As the previous section discussed, traveling waves help to generate streaming across pressure nodes, and the regional distribution of the acoustic field results in strong peripheral streaming. To achieve this design, surface acoustic wave devices require a decrease in the aperture length of the IDT to enhance uneven distributed acoustic field. Coupled with the necessity for potent acoustic pressure, the single focused IDT emerges as an ideal design [97]. Such focused IDTs possess narrow aperture widths to ensure the creation of unevenly distributed acoustic fields, while their curved design with gradually increased electrode sizes allows for the generation of substantial acoustic pressures. A single focused IDT, when paired with an appropriately designed channel, can induce vortex streaming. This streaming leverages the mechanism of inertial flow, which is usually facilitated by long circulating channels, to enable the differential separation of large and small particles (Figure 4A) [98]. A similar mechanism for separation can also be achieved by a bulk wave resonator, which can generate three-dimensional acoustic vortex streaming in a higher channel (Figure 4B) [99]. Bulk wave acoustic streaming also takes advantage of acoustic holography, where a patterned solid structure imposes phase changes on a uniformly initiated acoustic field, transforming it into a non-uniform pattern and consequently leading to intricate acoustic streaming configurations [100,101]. Normally, holographic bulk wave devices contain a transducer at the bottom with a holographic pattern cover for phase adjustment, resulting in the modified acoustic wave propagating in the liquid chamber above to generate acoustic streaming [102]. By altering the holographic pattern, this technology can cluster particles at the liquid’s surface via vertical vortex streaming (Figure 4C) [103]. Various strategies exist to mitigate the interference of acoustic radiation force on acoustic streaming-based manipulation. These include reducing the area of the acoustic field using focused IDTs, creating complex acoustic pressure distributions through phase adjustments, and utilizing resonators to generate intricate acoustic fields that favor acoustic streaming. However, a key challenge in this approach is achieving selective manipulation. Unlike acoustic radiation force, the drag force generated by acoustic streaming exhibits less variability with changes in particle size. Incorporating advancements from passive microfluidics, acoustic streaming technology has demonstrated a variety of methods for employing complex microstreaming patterns to achieve selectivity. Deriving robot-assisted control, which uses robot arms for controlling an acoustic streaming generating transducer, is another strategy for achieving selectivity on targets [104]. Since acoustic streaming manipulation provides an efficient approach for deriving multiple functions including noncontact trapping, pumping, and mixing, developing high selectivity would be beneficial to enhance the effectiveness of future acoustic streaming devices.
 |
Figure 4 Acoustic manipulation achieved by acoustic streaming. (A) Lateral vortices generated particle trapping and separation effect, large particles are trapped by the vortices, while small particles migrated to side of the channel by inertial flow effect [98]. (B) Vertical vortices generated by acoustic resonator achieve particle separation, scale bar: 100 μm [99]. (C) Acoustic hologram derives a patterned acoustic field through programmed surface design, the patterned acoustic field generates vortex to trap particles in the center [103]. (D) Digital acoustofluidics by SAW generated vortices, by programing the activation of IDTs in the array droplet on the surface could be moved [94]. (E) Digital acoustofluidics achieved by bluk acoustic wave for flexible droplet manipulation [95]. (F) Zebrafish imaging system integrated with acoustofludic manipulation, where acoustic streaming provides fish trapping and lateral rotation for surrounding imaging [26]. |
In addition to efforts to derive phase-changing acoustic waves, significant advancements have also been made particularly in active and real-time acoustic streaming-based manipulation. Digital acoustofluidics was introduced on a SAW platform to enable precise control (Figure 4D) [94]. This digital SAW platform employs an array of IDTs placed beneath an oil pool. The excitation of IDTs generates a vertical leakage wave in the oil layer, which in turn produces horizontal vortices on the oil surface. By selectively activating IDTs in the array, particles or droplets on the oil surface are directed toward an equilibrium point between adjacent IDTs and then moved past the point by aligning the excitation of IDTs towards the desired direction. A similar approach using vortex-based manipulation can be achieved on bulk acoustic wave platforms (Figure 4E) [95]. Here, four vertical transducers are arranged in a square configuration to serve as the basic unit, where joint streaming from the transducers creates vortices, allowing objects to be moved among hydrodynamic traps formed by symmetrical pairs of vortices. These digital manipulation devices achieve contactless control over objects, facilitating processes such as droplet mixing and fusion, essential for handling reagents, thus making them potent tools for chemical and biological experimental platforms. Furthermore, IDT vortex technology is also capable of in-channel object manipulation for imaging purposes. By installing an IDT at the bottom of a microfluidic channel, the leakage wave-induced acoustic streaming can generate a transverse vortex, enabling both object trapping and rotation (Figure 4F) [26]. With precise rotational control and the advanced biocompatibility of the acoustofluidic platform, this device achieves high-resolution imaging, such as in zebrafish, enabling the reconstruction of precise 3D images through rotational scanning. These endeavors highlight the versatility of acoustic streaming, which includes programmability, the ability to manipulate large objects, and the capacity to create extensive structured fields for various applications. In this branch of acoustic streaming technology, since the manipulation involves visible objects significantly larger than the acoustic field wavelengths, the effect of the acoustic radiation force can generally be disregarded. A promising direction for improvement lies in enhancing the spatial resolution of digital acoustofluidics. Given the fluid’s continuity, acoustic streaming generated by a portion of the platform can affect a wide area. Minimizing disruption from distant transducers can lead to a reduction in the overall size of the platform and enable the simultaneous manipulation of a large number of droplets, aligning the technology more closely with practical application requirements. Applying machine learning technology for transducer control could be a potential solution, since its capabilities on responses to complex phenomena occurred during multiple object manipulation [105].
INTEGRATION ACOUSTIC MANIPULATION WITH DROPLET MICROFLUIDICS
Acoustic manipulation has been employed in droplet microfluidic systems for several decades. Due to its small scale and high precision, droplet microfluidics has seen widespread use. In bulk systems containing complex constituents, desired reactions or signals from targets could be obscured or interfered with by noise from other components [106]. Droplet generation facilitates physical separation, resulting in purification, since each droplet may contain only a single molecule or cell [107]. This reduction in volume also lowers the detection threshold, especially for fluorescent signals, which are commonly utilized in experiments involving antibody reactions [108], probe detection [109], and gene expression [110]. Owing to these advantages, droplet microfluidics has found commercial applications in technologies such as digital droplet PCR [111], single-cell culture-based drug screening [112], and chemical reaction optimization [113]. Initially, acoustic manipulation was applied to droplet sorting, a process typically achieved through the trapping or propelling effects of acoustic radiation force or acoustic streaming (Figure 5A) [114–116]. Compared with conventional electrical and optical droplet sorting methods, the acoustic manipulation approach demonstrates a significant advantage in reducing heat generation, which can lead to enhanced biocompatibility and minimal thermal perturbations.
 |
Figure 5 Acoustofluidics manipulation applied in droplet microfluidics. (A) Droplet sorting by acoustic radiation force manipulation [114]. (B) Droplet splitting by acoustic streaming caused disruption [120]. (C) Droplet merge controlled by acoustic streaming caused surface disruption [121]. (D) Selective reagent and cell injection by acoustic streaming derived surface disruption [122]. (E) Droplet generation by acoustic streaming, which provide both surface disruption and pumping functions [123]. (F) Internal particle concentration and washing achieved by acoustic radiation trapping [128]. (G) Internal particle focusing and fixing in cross-linked hydrogel [129]. |
Beyond mere droplet manipulation, the real potential of acoustofluidics in droplet microfluidics lies in its capacity for surface disruption and non-contact internal manipulation. Droplet microfluidics requires at least one hydrophilic and one hydrophobic phase to form a discontinuous droplet phase and a continuous phase; therefore, surfactants are added to stabilize the emulsion system [117]. These amphipathic molecules reduce interfacial tension to form stable droplets. However, such stabilization hinders the discontinuous fusion necessary for injection and fusion applications, which allow for the addition of reagents and the initiation of reactions [118,119]. Acoustofludics has achieved fusion and splitting of droplet by the disruption derived by acoustic streaming (Figure 5B and C) [120,121]. A mechanism capable of disrupting the surfactant-stabilized surface is thus required. Surface acoustic waves (SAW) facilitate surface disruption primarily through acoustic streaming, which, by generating leakage wave-induced vertical microstreaming, enables reagent injection into droplets (Figure 5D) [122]. Focused IDT-generated acoustic streaming has also been employed for droplet generation, where horizontal acoustic streaming from a traveling wave provides adjustable pumping, allowing for specific barcoding of each droplet (Figure 5E) [123]. Furthermore, acoustic manipulation integrates droplet trapping with surface disruption to fuse two adjacent droplets within a channel [124,125]. The non-contact nature of acoustic manipulation is instrumental in droplet mixing or internal particle control; it accelerates reactions within a droplet by inducing an internal vortex that prevents droplet fusion or breakage [126,127]. Particle control within droplets is achieved through acoustic radiation force focusing, wherein particles are concentrated at the pressure node during droplet splitting, resulting in the formation of droplets with and without particles (Figure 5F and G) [128,129]. Nonetheless, the conflicting effects of acoustic streaming-induced surface disruption and acoustic radiation force-derived particle manipulation necessitate further investigation to elucidate the dominant mechanism in each scenario. Understanding these mechanisms will be crucial for optimizing the entire system and provide clues for applying acoustofluidic technology on more complicated systems include double emulsion system, multi-core double emulsion system, and hydrogel microcapsule system.
PRGORESS ON COUPLING ACOUSTIC RADIATION FORCE AND STREAMING
Although acoustic radiation force and acoustic streaming generally cause interference in most situations, recent years have seen efforts to couple these two effects. One such endeavor is the acoustic hologram base particle patterning, which uses incident acoustic wave to deform the liquid-air surface and generate acoustic pressure crests, while the acoustic streaming transport particles and cells to the acoustic pressure crests for forming stable patterning (Figure 6A) [130]. Another attempt is the wave-pillar excitation resonance platform. Unlike conventional surface acoustic wave (SAW) nanoparticle separation devices that create a continuous acoustic field in the channel, this platform generates an array of virtual acoustic wave pillars using resonance between the microfluidic channel and tunable SAWs (Figure 6B) [131]. The virtual acoustic wave pillars produce high acoustic pressure areas at the channel’s center, with lower acoustic pressure near the gaps between the pillars and the channel walls. This virtual pillar array mimics the effects of actual pillar arrays used to generate deterministic lateral displacement effects, which employ the array to produce lateral streaming and achieve separation based on lateral displacement differences contingent upon particle sizes [13,132]. The size-based separation of the virtual acoustic wave pillar platform is accomplished through fluidic forces generated by the streaming flow around the virtual pillars and the obstacle effect from the areas of high acoustic radiation force. Although this platform does not utilize conventional acoustic streaming, it represents a significant attempt to harness the coupling effects of streaming influenced by acoustic waves and the acoustic radiation force. This method employs acoustic streaming for the long-distance movement of particles and utilizes focused acoustic radiation generated by reflection from a raised flow nest. It demonstrates that by differentiating the areas affected, it is possible to harness both effects in a single device without interference.
 |
Figure 6 Acoustofluidics manipulation with coupled acoustic radiation force and streaming. (A) Acoustofludics holographic manipulation with patterned acoustic field generated pressure crests for acoustic radiation force patterning, meanwhile acoustic streaming achieve particle transportation to the pressure crests [130]. (B) Wave-pillar excitation resonance derived virtual pillar array for deterministic lateral displacement-based separation, smaller particles can pass the virtual pillar with straight streaming line, while large particles are moved to edge areas, scale bar: 20 μm [131]. (C) Droplet in acoustic field presents both acoustic streaming based vortex and acoustic radiation force derived concentration, resulting ring patterning of particles [53]. (D) Acoustofluidics centrifugation achieved by coupling of acoustic streaming derived vortex and acoustic radiation force derived concentration, the coupling of the two effects enables a complicated moving trajectory of particle and nanometer sized particle separation, scale bar: 500 μm [133]. |
The coupling of acoustic radiation force with acoustic streaming has been realized in the acoustofluidic centrifugation platform, which utilizes two surface acoustic waves (SAWs) propagating in opposite directions to act on the two flanks of a droplet [133]. While previous devices could drive droplet vortex [134] or generate in-droplet vortices and acoustic radiation force derived ring-pattern structures simultaneously (Figure 6C) [53], this platform employs slanted interdigital transducers (IDTs) with varied finger spacing across the transducer widths (Figure 6D). The implementation of slanted IDTs permits frequency adjustments and the alteration of the IDT’s excitation area. Consequently, the SAW direction can be fine-tuned to optimize the coupling between the internal vortex and the internal acoustic pressure area. The acoustic pressure and centrifugal force counterbalance the surface tension of the droplet, resulting in a stable spinning mode characterized by periodic rotational boundary deformation. Internal particles are induced to migrate toward the droplet’s center along a dual-axis rotational trajectory due to the combined effect of acoustic radiation force and the drag force from acoustic streaming. This synergetic effect enables the screening of particles smaller than 100 nm with a resolution of 10 nm, surpassing previously established parameters for acoustofluidic devices. Beyond particle separation, this platform is also adept at leveraging the coupling effect to simultaneously achieve concentration and lysis. These accomplishments underscore the substantial potential of combining acoustic radiation force and acoustic streaming. However, the coupling of acoustic radiation force and acoustic streaming entails complex phenomena, including droplet deformation, resonance of the acoustic wave within the droplet, and intricate particle movement. To realize these coupled effects on other platforms, advancements are needed in both the investigation of underlying mechanisms and the development of simulation models.
SUMMARY
Having demonstrated significant potential for manipulation in clinical and research settings, acoustofluidics has evolved into a more specialized and functional platform. Depending on the fundamental manipulation mechanisms, it can be categorized into two types: acoustic radiation force and acoustic streaming manipulation. Due to the generation mechanism, acoustic radiation force shows a high resolution on distinguishing particle sizes and densities differences, enabling the technology to derive precise and small-scale manipulation. Such features are important in small particle separation, flexible manipulation, and mechanical properties evaluation. Recent research on using acoustic radiation force to select single bacterial with different density because of genetical differences [135], viability related cellular compressibility evaluation [136], and numerous research on cell cluster construction for drug screen and developmental biological study purpose [137,138], demonstrate this feasibility on precise screening and analysis. However, since the accuracy of the mechanism, inhibition disruption from acoustic streaming has become an obstacle for investigating the potential of acoustic radiation force-based devices. In contrast, acoustic streaming offers advantages in terms of strength and effect range, making it an ideal approach for large-scale manipulation and long-term stimulation. While this mechanism could avoid acoustic radiation force interference by larging the scale of the system, spatial resolution becomes a concern since the requirement from microstreaming based microreactor and drug delivery studies [139,140]. Based on the complexity on the fundamental mechanism of acoustofluidic manipulation, further investigation should be carried out on interference inhibition, performance on complex fluid systems, and with attempts to harness both effects for enhanced manipulation. Such progress will benefit the acoustofluidic technology to be used for real-case applications.
Data availability
The original data are available from corresponding authors upon reasonable request.
Funding
This work was supported by the National Natural Science Foundation of China (52025055, 52322513, and 51975467), and the Shaanxi University Youth Innovation Team.
Author contributions
Z.W. originated and wrote the review. J.X. provided support on checking the theory and mechanism. R.G. provided support on droplet microfluidics review. D.A.W. and X.L. provided quality check.
Conflict of interest
The authors declare no conflict of interest.
References
- Johnson TJ, Ross D, Locascio LE. Rapid microfluidic mixing. Anal Chem 2002; 74: 45-51. [Article] [Google Scholar]
- Lenshof A, Laurell T. Continuous separation of cells and particles in microfluidic systems. Chem Soc Rev 2010; 39: 1203-1217. [Article] [CrossRef] [PubMed] [Google Scholar]
- Karnik R, Gu F, Basto P, et al. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett 2008; 8: 2906-2912. [Article] [Google Scholar]
- Ng AHC, Uddayasankar U, Wheeler AR. Immunoassays in microfluidic systems. Anal Bioanal Chem 2010; 397: 991-1007. [Article] [Google Scholar]
- Chen X, Cui D, Liu C, et al. Continuous flow microfluidic device for cell separation, cell lysis and DNA purification. Anal Chim Acta 2007; 584: 237-243. [Article] [Google Scholar]
- Kumemura M, Collard D, Yamahata C, et al. Single DNA molecule isolation and trapping in a microfluidic device. ChemPhysChem 2007; 8: 1875-1880. [Article] [CrossRef] [PubMed] [Google Scholar]
- Autebert J, Coudert B, Bidard FC, et al. Microfluidic: An innovative tool for efficient cell sorting. Methods 2012; 57: 297-307. [Article] [PubMed] [Google Scholar]
- Lee CY, Wang WT, Liu CC, et al. Passive mixers in microfluidic systems: A review. Chem Eng J 2016; 288: 146-160. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Amini H, Lee W, Di Carlo D. Inertial microfluidic physics. Lab Chip 2014; 14: 2739-2761. [Article] [Google Scholar]
- Zhu P, Wang L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017; 17: 34-75. [Article] [Google Scholar]
- Bayareh M. An updated review on particle separation in passive microfluidic devices. Chem Eng Process-Process Intensif 2020; 153: 107984. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Di Carlo D, Edd JF, Irimia D, et al. Equilibrium separation and filtration of particles using differential inertial focusing. Anal Chem 2008; 80: 2204-2211. [Article] [Google Scholar]
- Salafi T, Zhang Y, Zhang Y. A review on deterministic lateral displacement for particle separation and detection. Nano-Micro Lett 2019; 11: 1-33. [Article] [Google Scholar]
- Hou HW, Bhagat AAS, Lee WC, et al. Microfluidic devices for blood fractionation. Micromachines 2011; 2: 319-343. [Article] [Google Scholar]
- Nivedita N, Ligrani P, Papautsky I. Dean flow dynamics in low-aspect ratio spiral microchannels. Sci Rep 2017; 7: 44072. [Article] [Google Scholar]
- Zhang J, Yan S, Yuan D, et al. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016; 16: 10-34. [Article] [Google Scholar]
- Martel JM, Toner M. Inertial focusing in microfluidics. Annu Rev Biomed Eng 2014; 16: 371-396. [Article] [Google Scholar]
- Wang X, Chen S, Kong M, et al. Enhanced cell sorting and manipulation with combined optical tweezer and microfluidic chip technologies. Lab Chip 2011; 11: 3656-3662. [Article] [Google Scholar]
- Dorfman KD, King SB, Olson DW, et al. Beyond gel electrophoresis: Microfluidic separations, fluorescence burst analysis, and DNA stretching. Chem Rev 2013; 113: 2584-2667. [Article] [CrossRef] [PubMed] [Google Scholar]
- Gijs MAM, Lacharme F, Lehmann U. Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem Rev 2010; 110: 1518-1563. [Article] [CrossRef] [PubMed] [Google Scholar]
- Friend J, Yeo LY. Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics. Rev Mod Phys 2011; 83: 647-704. [Article] [Google Scholar]
- Wu M, Ozcelik A, Rufo J, et al. Acoustofluidic separation of cells and particles. Microsyst Nanoeng 2019; 5: 32. [Article] [NASA ADS] [PubMed] [Google Scholar]
- Rufo J, Cai F, Friend J, et al. Acoustofluidics for biomedical applications. Nat Rev Methods Primers 2022; 2: 30. [Article] [Google Scholar]
- Wiklund M. Acoustofluidics 12: Biocompatibility and cell viability in microfluidic acoustic resonators. Lab Chip 2012; 12: 2018-2028. [Article] [Google Scholar]
- Zhang P, Chen C, Guo F, et al. Contactless, programmable acoustofluidic manipulation of objects on water. Lab Chip 2019; 19: 3397-3404. [Article] [Google Scholar]
- Chen C, Gu Y, Philippe J, et al. Acoustofluidic rotational tweezing enables high-speed contactless morphological phenotyping of zebrafish larvae. Nat Commun 2021; 12: 1118. [Article] [Google Scholar]
- Wang Z, Li F, Rufo J, et al. Acoustofluidic salivary exosome isolation. J Mol Diagnost 2020; 22: 50-59. [Article] [Google Scholar]
- Wu M, Ouyang Y, Wang Z, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci USA 2017; 114: 10584-10589. [Article] [Google Scholar]
- Gu Y, Chen C, Wang Z, et al. Plastic-based acoustofluidic devices for high-throughput, biocompatible platelet separation. Lab Chip 2019; 19: 394-402. [Article] [Google Scholar]
- Antfolk M, Magnusson C, Augustsson P, et al. Acoustofluidic, label-free separation and simultaneous concentration of rare tumor cells from white blood cells. Anal Chem 2015; 87: 9322-9328. [Article] [Google Scholar]
- Wang Z, Wang H, Becker R, et al. Acoustofluidic separation enables early diagnosis of traumatic brain injury based on circulating exosomes. Microsyst Nanoeng 2021; 7: 20. [Article] [NASA ADS] [PubMed] [Google Scholar]
- Connacher W, Zhang N, Huang A, et al. Micro/nano acoustofluidics: Materials, phenomena, design, devices, and applications. Lab Chip 2018; 18: 1952-1996. [Article] [Google Scholar]
- Li P, Huang TJ. Applications of acoustofluidics in bioanalytical chemistry. Anal Chem 2018; 91: 757-767. [Article] [Google Scholar]
- Fakhfouri A, Devendran C, Albrecht T, et al. Surface acoustic wave diffraction driven mechanisms in microfluidic systems. Lab Chip 2018; 18: 2214-2224. [Article] [Google Scholar]
- Zhang SP, Lata J, Chen C, et al. Digital acoustofluidics enables contactless and programmable liquid handling. Nat Commun 2018; 9: 2928. [Article] [Google Scholar]
- Park J, Destgeer G, Afzal M, et al. Acoustofluidic generation of droplets with tunable chemical concentrations. Lab Chip 2020; 20: 3922-3929. [Article] [Google Scholar]
- Duck FA. Radiation pressure and acoustic streaming. In: Ultrasound in Medicine. Boca Raton: CRC Press, 1998, 39-56 [Google Scholar]
- Sadhal SS. Acoustofluidics 13: Analysis of acoustic streaming by perturbation methods. Lab Chip 2012; 12: 2292-2300. [Article] [Google Scholar]
- Bruus H. Acoustofluidics 7: The acoustic radiation force on small particles. Lab Chip 2012; 12: 1014-1021. [Article] [Google Scholar]
- Wiklund M, Green R, Ohlin M. Acoustofluidics 14: Applications of acoustic streaming in microfluidic devices. Lab Chip 2012; 12: 2438-2451. [Article] [Google Scholar]
- Ahmed D, Mao X, Shi J, et al. A millisecond micromixer via single-bubble-based acoustic streaming. Lab Chip 2009; 9: 2738-2741. [Article] [Google Scholar]
- Nama N, Huang PH, Huang TJ, et al. Investigation of acoustic streaming patterns around oscillating sharp edges. Lab Chip 2014; 14: 2824-2836. [Article] [Google Scholar]
- Cai H, Ao Z, Wu Z, et al. Profiling cell-matrix adhesion using digitalized acoustic streaming. Anal Chem 2019; 92: 2283-2290. [Article] [Google Scholar]
- Bruus H. Theoretical Microfluidics. Oxford: Oxford University Press, 2007 [Google Scholar]
- Ding X, Li P, Lin SCS, et al. Surface acoustic wave microfluidics. Lab Chip 2013; 13: 3626-3649. [Article] [Google Scholar]
- Bruus H. Acoustofluidics 2: Perturbation theory and ultrasound resonance modes. Lab Chip 2012; 12: 20-28. [Article] [Google Scholar]
- Bremond N, Arora M, Ohl CD, et al. Controlled multibubble surface cavitation. Phys Rev Lett 2006; 96: 224501. [Article] [Google Scholar]
- Costanzo F, Gray GL, Andia PC. On the definitions of effective stress and deformation gradient for use in MD: Hill’s macro-homogeneity and the virial theorem. Int J Eng Sci 2005; 43: 533-555. [Article] [Google Scholar]
- Andia PC, Costanzo F, Gray GL. A Lagrangian-based continuum homogenization approach applicable to molecular dynamics simulations. Int J Solids Struct 2005; 42: 6409-6432. [Article] [Google Scholar]
- Doinikov AA. Acoustic radiation forces: Classical theory and recent advances. In: Recent Research Developments in Acoustics. Trivandrum: Transworld Research Network, 2003 [Google Scholar]
- Landau LD, Lifshitz EM. Course of Theoretical Physics. 3rd ed. Oxford: Elsevier, 2013 [Google Scholar]
- Loudet JC, Hanusse P, Poulin P. Stokes drag on a sphere in a nematic liquid crystal. Science 2004; 306: 1525. [Article] [Google Scholar]
- Destgeer G, Ha B, Park J, et al. Lamb wave-based acoustic radiation force-driven particle ring formation inside a sessile droplet. Anal Chem 2016; 88: 3976-3981. [Article] [Google Scholar]
- Hagsäter SM, Jensen TG, Bruus H, et al. Acoustic resonances in microfluidic chips: Full-image micro-PIV experiments and numerical simulations. Lab Chip 2007; 7: 1336-1344. [Article] [Google Scholar]
- Bora M, Shusteff M. Efficient coupling of acoustic modes in microfluidic channel devices. Lab Chip 2015; 15: 3192-3202. [Article] [Google Scholar]
- Lenshof A, Evander M, Laurell T, et al. Acoustofluidics 5: Building microfluidic acoustic resonators. Lab Chip 2012; 12: 684-695. [Article] [Google Scholar]
- Lenshof A, Magnusson C, Laurell T. Acoustofluidics 8: Applications of acoustophoresis in continuous flow microsystems. Lab Chip 2012; 12: 1210-1223. [Article] [Google Scholar]
- Yazdani AM, Şişman A. A novel numerical model to simulate acoustofluidic particle manipulation. Phys Scr 2020; 95: 095002. [Article] [Google Scholar]
- Namnabat MS, Moghimi Zand M, Houshfar E. 3D numerical simulation of acoustophoretic motion induced by boundary-driven acoustic streaming in standing surface acoustic wave microfluidics. Sci Rep 2021; 11: 13326. [Article] [Google Scholar]
- Lei J, Cheng F, Li K, et al. Numerical simulation of continuous separation of microparticles in two-stage acousto-microfluidic systems. Appl Math Model 2020; 83: 342-356. [Article] [MathSciNet] [Google Scholar]
- Liu P, Tian Z, Yang K, et al. Acoustofluidic black holes for multifunctional in-droplet particle manipulation. Sci Adv 2022; 8: eabm2592. [Article] [Google Scholar]
- Maramizonouz S, Jia C, Rahmati M, et al. Acoustofluidic patterning inside capillary tubes using standing surface acoustic waves. Int J Mech Sci 2022; 214: 106893. [Article] [Google Scholar]
- Gao Y, Wu M, Lin Y, et al. Acoustic microfluidic separation techniques and bioapplications: A review. Micromachines 2020; 11: 921. [Article] [PubMed] [Google Scholar]
- Wu M, Chen K, Yang S, et al. High-throughput cell focusing and separation via acoustofluidic tweezers. Lab Chip 2018; 18: 3003-3010. [Article] [Google Scholar]
- Xie Y, Mao Z, Bachman H, et al. Acoustic cell separation based on density and mechanical properties. J BioMech Eng 2020; 142: 031005. [Article] [PubMed] [Google Scholar]
- Wu M, Chen C, Wang Z, et al. Separating extracellular vesicles and lipoproteins via acoustofluidics. Lab Chip 2019; 19: 1174-1182. [Article] [Google Scholar]
- Mao Z, Li P, Wu M, et al. Enriching nanoparticles via acoustofluidics. ACS Nano 2017; 11: 603-612. [Article] [Google Scholar]
- Wu M, Huang P, Zhang R, et al. Circulating tumor cell phenotyping via high-throughput acoustic separation. Small 2018; 14: 1801131. [Article] [PubMed] [Google Scholar]
- Weser R, Winkler A, Weihnacht M, et al. The complexity of surface acoustic wave fields used for microfluidic applications. Ultrasonics 2020; 106: 106160. [Article] [Google Scholar]
- Weser R, Darinskii AN, Weihnacht M, et al. Experimental and numerical investigations of mechanical displacements in surface acoustic wave bounded beams. Ultrasonics 2020; 106: 106077. [Article] [Google Scholar]
- Fakhfouri A, Devendran C, Ahmed A, et al. The size dependant behaviour of particles driven by a travelling surface acoustic wave (TSAW). Lab Chip 2018; 18: 3926-3938. [Article] [Google Scholar]
- Devendran C, Collins DJ, Neild A. The role of channel height and actuation method on particle manipulation in surface acoustic wave (SAW)-driven microfluidic devices. Microfluid Nanofluid 2022; 26: 9. [Article] [Google Scholar]
- Kolesnik K, Hashemzadeh P, Peng D, et al. Periodic Rayleigh streaming vortices and Eckart flow arising from traveling-wave-based diffractive acoustic fields. Phys Rev E 2021; 104: 045104. [Article] [Google Scholar]
- Song S, Wang Q, Zhou J, et al. Design of interdigitated transducers for acoustofluidic applications. Nanotechnol Precision Eng 2022; 5: 035001. [Article] [Google Scholar]
- Martin G, Chen D. Diffraction analysis of slanted-finger interdigital transducers. IEEE Trans Microwave Theor Techn 2001; 49: 838-843. [Article] [NASA ADS] [Google Scholar]
- Zhao S, Wu M, Yang S, et al. A disposable acoustofluidic chip for nano/microparticle separation using unidirectional acoustic transducers. Lab Chip 2020; 20: 1298-1308. [Article] [Google Scholar]
- He C, Ni X, Ge H, et al. Acoustic topological insulator and robust one-way sound transport. Nat Phys 2016; 12: 1124-1129. [Article]arxiv:1512.03273 [Google Scholar]
- Xia JP, Jia D, Sun HX, et al. Programmable coding acoustic topological insulator. Adv Mater 2018; 30: 1805002. [Article] [PubMed] [Google Scholar]
- Mamishev AV, Sundara-Rajan K, Fumin Yang K, et al. Interdigital sensors and transducers. Proc IEEE 2004; 92: 808-845. [Article] [Google Scholar]
- Shi J, Ahmed D, Mao X, et al. Acoustic tweezers: Patterning cells and microparticles using standing surface acoustic waves (SSAW). Lab Chip 2009; 9: 2890-2895. [Article] [Google Scholar]
- Tian Z, Yang S, Huang PH, et al. Wave number-spiral acoustic tweezers for dynamic and reconfigurable manipulation of particles and cells. Sci Adv 2019; 5: eaau6062. [Article] [Google Scholar]
- Kang P, Tian Z, Yang S, et al. Acoustic tweezers based on circular, slanted-finger interdigital transducers for dynamic manipulation of micro-objects. Lab Chip 2020; 20: 987-994. [Article] [Google Scholar]
- Ding X, Lin SCS, Kiraly B, et al. On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves. Proc Natl Acad Sci USA 2012; 109: 11105-11109. [Article] [Google Scholar]
- Yang S, Tian Z, Wang Z, et al. Harmonic acoustics for dynamic and selective particle manipulation. Nat Mater 2022; 21: 540-546. [Article] [Google Scholar]
- Ao Z, Cai H, Wu Z, et al. Controllable fusion of human brain organoids using acoustofluidics. Lab Chip 2021; 21: 688-699. [Article] [Google Scholar]
- Wu Z, Chen B, Wu Y, et al. Scaffold-free generation of heterotypic cell spheroids using acoustofluidics. Lab Chip 2021; 21: 3498-3508. [Article] [Google Scholar]
- Wu Y, Zhao Y, Islam K, et al. Acoustofluidic engineering of functional vessel-on-a-chip. ACS BioMater Sci Eng 2023; 9: 6273-6281. [Article] [Google Scholar]
- Jeger-Madiot N, Arakelian L, Setterblad N, et al. Self-organization and culture of Mesenchymal Stem Cell spheroids in acoustic levitation. Sci Rep 2021; 11: 8355. [Article] [Google Scholar]
- Wu Z, Ao Z, Cai H, et al. Acoustofluidic assembly of primary tumor-derived organotypic cell clusters for rapid evaluation of cancer immunotherapy. J Nanobiotechnol 2023; 21: 40. [Article] [Google Scholar]
- Cai H, Ao Z, Hu L, et al. Acoustofluidic assembly of 3D neurospheroids to model Alzheimer’s disease. Analyst 2020; 145: 6243-6253. [Article] [Google Scholar]
- Boluriaan S, Morris PJ. Acoustic streaming: From rayleigh to today. Int J Aeroacoust 2003; 2: 255-292. [Article] [Google Scholar]
- Wei W, Wang Y, Wang Z, et al. Microscale acoustic streaming for biomedical and bioanalytical applications. TrAC Trends Anal Chem 2023; 160: 116958. [Article] [Google Scholar]
- Wu J. Acoustic streaming and its applications. Fluids 2018; 3: 108. [Article] [Google Scholar]
- Zhang P, Chen C, Su X, et al. Acoustic streaming vortices enable contactless, digital control of droplets. Sci Adv 2020; 6: eaba0606. [Article] [Google Scholar]
- Zhu H, Zhang P, Zhong Z, et al. Acoustohydrodynamic tweezers via spatial arrangement of streaming vortices. Sci Adv 2021; 7: eabc7885. [Article] [Google Scholar]
- Draz MS, Dupouy D, Gijs MAM. Acoustofluidic large-scale mixing for enhanced microfluidic immunostaining for tissue diagnostics. Lab Chip 2023; 23: 3258-3271. [Article] [Google Scholar]
- O’Rorke R, Winkler A, Collins D, et al. Slowness curve surface acoustic wave transducers for optimized acoustic streaming. RSC Adv 2020; 10: 11582-11589. [Article] [Google Scholar]
- Collins DJ, Ma Z, Han J, et al. Continuous micro-vortex-based nanoparticle manipulation via focused surface acoustic waves. Lab Chip 2017; 17: 91-103. [Article] [Google Scholar]
- Yang Y, Pang W, Zhang H, et al. Manipulation of single cells via a stereo acoustic streaming tunnel (SteAST). Microsyst Nanoeng 2022; 8: 88. [Article] [NASA ADS] [PubMed] [Google Scholar]
- Hildebrand BP. An Introduction to Acoustical Holography. New York: Springer Science & Business Media, 2013 [Google Scholar]
- Melde K, Mark AG, Qiu T, et al. Holograms for acoustics. Nature 2016; 537: 518-522. [Article] [Google Scholar]
- Ma Z, Holle AW, Melde K, et al. Acoustic holographic cell patterning in a biocompatible hydrogel. Adv Mater 2020; 32: 1904181. [Article] [PubMed] [Google Scholar]
- Li J, Crivoi A, Peng X, et al. Three dimensional acoustic tweezers with vortex streaming. Commun Phys 2021; 4: 113. [Article] [NASA ADS] [Google Scholar]
- Durrer J, Agrawal P, Ozgul A, et al. A robot-assisted acoustofluidic end effector. Nat Commun 2022; 13: 6370. [Article] [Google Scholar]
- Yiannacou K, Sariola V. Controlled manipulation and active sorting of particles inside microfluidic chips using bulk acoustic waves and machine learning. Langmuir 2021; 37: 4192-4199. [Article] [Google Scholar]
- Teh SY, Lin R, Hung LH, et al. Droplet microfluidics. Lab Chip 2008; 8: 198-220. [Article] [Google Scholar]
- Chou WL, Lee PY, Yang CL, et al. Recent advances in applications of droplet microfluidics. Micromachines 2015; 6: 1249-1271. [Article] [Google Scholar]
- Guo MT, Rotem A, Heyman JA, et al. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012; 12: 2146-2155. [Article] [Google Scholar]
- Zhu Z, Jenkins G, Zhang W, et al. Single-molecule emulsion PCR in microfluidic droplets. Anal Bioanal Chem 2012; 403: 2127-2143. [Article] [Google Scholar]
- Rakszewska A, Tel J, Chokkalingam V, et al. One drop at a time: Toward droplet microfluidics as a versatile tool for single-cell analysis. NPG Asia Mater 2014; 6: e133. [Article] [Google Scholar]
- Byrnes SA, Chang TC, Huynh T, et al. Simple polydisperse droplet emulsion polymerase chain reaction with statistical volumetric correction compared with microfluidic droplet digital polymerase chain reaction. Anal Chem 2018; 90: 9374-9380. [Article] [Google Scholar]
- Terekhov SS, Smirnov IV, Stepanova AV, et al. Microfluidic droplet platform for ultrahigh-throughput single-cell screening of biodiversity. Proc Natl Acad Sci USA 2017; 114: 2550-2555. [Article] [Google Scholar]
- Baumgartner LM, Coley CW, Reizman BJ, et al. Optimum catalyst selection over continuous and discrete process variables with a single droplet microfluidic reaction platform. React Chem Eng 2018; 3: 301-311. [Article] [Google Scholar]
- Li S, Ding X, Guo F, et al. An on-chip, multichannel droplet sorter using standing surface acoustic waves. Anal Chem 2013; 85: 5468-5474. [Article] [Google Scholar]
- Lee C, Lee J, Kim HH, et al. Microfluidic droplet sorting with a high frequency ultrasound beam. Lab Chip 2012; 12: 2736-2742. [Article] [Google Scholar]
- Qin X, Wei X, Li L, et al. Acoustic valves in microfluidic channels for droplet manipulation. Lab Chip 2021; 21: 3165-3173. [Article] [Google Scholar]
- Baret JC. Surfactants in droplet-based microfluidics. Lab Chip 2012; 12: 422-433. [Article] [Google Scholar]
- Mazutis L, Griffiths AD. Selective droplet coalescence using microfluidic systems. Lab Chip 2012; 12: 1800-1806. [Article] [Google Scholar]
- Rhee M, Light YK, Yilmaz S, et al. Pressure stabilizer for reproducible picoinjection in droplet microfluidic systems. Lab Chip 2014; 14: 4533-4539. [Article] [Google Scholar]
- Park J, Jung JH, Park K, et al. On-demand acoustic droplet splitting and steering in a disposable microfluidic chip. Lab Chip 2018; 18: 422-432. [Article] [Google Scholar]
- Sesen M, Alan T, Neild A. Microfluidic on-demand droplet merging using surface acoustic waves. Lab Chip 2014; 14: 3325-3333. [Article] [Google Scholar]
- Mutafopulos K, Lu PJ, Garry R, et al. Selective cell encapsulation, lysis, pico-injection and size-controlled droplet generation using traveling surface acoustic waves in a microfluidic device. Lab Chip 2020; 20: 3914-3921. [Article] [Google Scholar]
- Zhang P, Wang W, Fu H, et al. Deterministic droplet coding via acoustofluidics. Lab Chip 2020; 20: 4466-4473. [Article] [Google Scholar]
- Bussiere V, Vigne A, Link A, et al. High-throughput triggered merging of surfactant-stabilized droplet pairs using traveling surface acoustic waves. Anal Chem 2019; 91: 13978-13985. [Article] [Google Scholar]
- Sesen M, Fakhfouri A, Neild A. Coalescence of surfactant-stabilized adjacent droplets using surface acoustic waves. Anal Chem 2019; 91: 7538-7545. [Article] [Google Scholar]
- Fornell A, Cushing K, Nilsson J, et al. Binary particle separation in droplet microfluidics using acoustophoresis. Appl Phys Lett 2018; 112: 063701. [Article] [Google Scholar]
- Duncanson WJ, Arriaga LR, Ung WL, et al. Microfluidic fabrication of perfluorohexane-shelled double emulsions for controlled loading and acoustic-triggered release of hydrophilic agents. Langmuir 2014; 30: 13765-13770. [Article] [Google Scholar]
- Park J, Destgeer G, Kim H, et al. In-droplet microparticle washing and enrichment using surface acoustic wave-driven acoustic radiation force. Lab Chip 2018; 18: 2936-2945. [Article] [Google Scholar]
- Fornell A, Pohlit H, Shi Q, et al. Acoustic focusing of beads and cells in hydrogel droplets. Sci Rep 2021; 11: 7479. [Article] [Google Scholar]
- Gu Y, Chen C, Rufo J, et al. Acoustofluidic holography for micro- to nanoscale particle manipulation. ACS Nano 2020; 14: 14635-14645. [Article] [Google Scholar]
- Zhang J, Chen C, Becker R, et al. A solution to the biophysical fractionation of extracellular vesicles: Acoustic nanoscale separation via wave-pillar excitation resonance (ANSWER). Sci Adv 2022; 8: eade0640. [Article] [Google Scholar]
- McGrath J, Jimenez M, Bridle H. Deterministic lateral displacement for particle separation: A review. Lab Chip 2014; 14: 4139-4158. [Article] [Google Scholar]
- Gu Y, Chen C, Mao Z, et al. Acoustofluidic centrifuge for nanoparticle enrichment and separation. Sci Adv 2021; 7: eabc0467. [Article] [Google Scholar]
- Destgeer G, Cho H, Ha BH, et al. Acoustofluidic particle manipulation inside a sessile droplet: Four distinct regimes of particle concentration. Lab Chip 2016; 16: 660-667. [Article] [Google Scholar]
- Wu D, Baresch D, Cook C, et al. Biomolecular actuators for genetically selective acoustic manipulation of cells. Sci Adv 2023; 9: eadd9186. [Article] [Google Scholar]
- Fu Q, Zhang Y, Huang T, et al. Measurement of cell compressibility changes during epithelial-mesenchymal transition based on acoustofluidic microdevice. Biomicrofluidics 2021; 15: 064101. [Article] [Google Scholar]
- He Y, Yang S, Liu P, et al. Acoustofluidic interfaces for the mechanobiological secretome of MSCs. Nat Commun 2023; 14: 7639. [Article] [Google Scholar]
- Richard C, Devendran C, Ashtiani D, et al. Acoustofluidic cell micro-dispenser for single cell trajectory control. Lab Chip 2022; 22: 3533-3544. [Article] [Google Scholar]
- Kim S, Nam H, Cha B, et al. Acoustofluidic stimulation of functional immune cells in a microreactor. Adv Sci 2022; 9: 2105809. [Article] [CrossRef] [Google Scholar]
- Salari A, Appak-Baskoy S, Coe IR, et al. Dosage-controlled intracellular delivery mediated by acoustofluidics for lab on a chip applications. Lab Chip 2021; 21: 1788-1797. [Article] [Google Scholar]
All Figures
 |
Figure 1 Mechanisms of acoustic manipulation include acoustic radiation force and acoustics streaming. (A) Basic design of acoustofluidic devices, the device using bulk acoustic wave (upside schematics) featured by acoustic wave propagating in the bulk of the liquid, while surface acoustic wave device (down side schematics) featured by the Rayleigh wave propagating in the surface of the substrate [22]. (B) The mechanism of acoustic radiation force is the scattering of acoustic wave on the particle’s surface, indicating that the particle is directly manipulated by the acoustic field [39]. (C) The generation of acoustic streaming derives from the compression of fluid within the acoustic field; particle manipulation is achieved by generated streaming [43]. (D) Schematics of the dominance of acoustic radiation force and acoustic streaming in particle manipulation, a same acoustofludic device generates both effects, normally smaller particles are manipulated by acoustic streaming effect (up), while larger particles are manipulated by acoustic radiation force [53]. (E) Practical analysis of in a same acoustic field, larger particles are dominant by acoustic radiation force (left) and smaller particles follows the pattern of acoustic streaming (right) [54]. |
| In the text | |
 |
Figure 2 Progress on acoustic radiation force-based subnano- and nanometer size particles manipulation. (A) Exosomes, a subpopulation of 30–200 nm macrovesicles, are separated from blood sample using double separation module with increased frequency SAW fields [28]. (B) The approach for generating lateral and vertical standing wave fields, lateral standing wave field is generated by opposite propagating traveling waves from double IDTs, and vertical standing wave field is generated by the leakage waves and reflected leakage waves from glass bottom of the channel [68]. (C) The high frequency IDT generates uneven acoustic field with the center area dominated by acoustic radiation force and edge areas dominated by acoustic streaming caused vortices, parameters including aperture length of IDT and channel structure can influence this uneven distribution (left), this phenomenon is caused by diffraction during acoustic wave propagation, which induces distribution deterioration with distance increasing [34]. (D) Utilization of unidirectional IDTs can generate stronger acoustic fields with less uneven distribution, indicating improvement on IDT design can avoid diffraction problems [76]. |
| In the text | |
 |
Figure 3 Progress on acoustic radiation force derived flexible manipulation. (A) Stable particles patterning with stand acoustic wave field [80]. (B) Circular, slanted-finger IDTs can generated adjustable directional acoustic waves by changing the frequency of excitation currency, change on frequency causes excitation area on the IDT changes, which cause propagation direction change and can achieve flexible manipulation [82]. (C) Harmonic IDTs composed of N individual transducers for harmonic SAWs derives selective and reversible assembly of colloidal crystals or cells by Fourier-synthesized harmonic waves [84]. |
| In the text | |
 |
Figure 4 Acoustic manipulation achieved by acoustic streaming. (A) Lateral vortices generated particle trapping and separation effect, large particles are trapped by the vortices, while small particles migrated to side of the channel by inertial flow effect [98]. (B) Vertical vortices generated by acoustic resonator achieve particle separation, scale bar: 100 μm [99]. (C) Acoustic hologram derives a patterned acoustic field through programmed surface design, the patterned acoustic field generates vortex to trap particles in the center [103]. (D) Digital acoustofluidics by SAW generated vortices, by programing the activation of IDTs in the array droplet on the surface could be moved [94]. (E) Digital acoustofluidics achieved by bluk acoustic wave for flexible droplet manipulation [95]. (F) Zebrafish imaging system integrated with acoustofludic manipulation, where acoustic streaming provides fish trapping and lateral rotation for surrounding imaging [26]. |
| In the text | |
 |
Figure 5 Acoustofluidics manipulation applied in droplet microfluidics. (A) Droplet sorting by acoustic radiation force manipulation [114]. (B) Droplet splitting by acoustic streaming caused disruption [120]. (C) Droplet merge controlled by acoustic streaming caused surface disruption [121]. (D) Selective reagent and cell injection by acoustic streaming derived surface disruption [122]. (E) Droplet generation by acoustic streaming, which provide both surface disruption and pumping functions [123]. (F) Internal particle concentration and washing achieved by acoustic radiation trapping [128]. (G) Internal particle focusing and fixing in cross-linked hydrogel [129]. |
| In the text | |
 |
Figure 6 Acoustofluidics manipulation with coupled acoustic radiation force and streaming. (A) Acoustofludics holographic manipulation with patterned acoustic field generated pressure crests for acoustic radiation force patterning, meanwhile acoustic streaming achieve particle transportation to the pressure crests [130]. (B) Wave-pillar excitation resonance derived virtual pillar array for deterministic lateral displacement-based separation, smaller particles can pass the virtual pillar with straight streaming line, while large particles are moved to edge areas, scale bar: 20 μm [131]. (C) Droplet in acoustic field presents both acoustic streaming based vortex and acoustic radiation force derived concentration, resulting ring patterning of particles [53]. (D) Acoustofluidics centrifugation achieved by coupling of acoustic streaming derived vortex and acoustic radiation force derived concentration, the coupling of the two effects enables a complicated moving trajectory of particle and nanometer sized particle separation, scale bar: 500 μm [133]. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

