| Issue |
Natl Sci Open
Volume 4, Number 6, 2025
Special Topic: Intelligent Materials and Devices
|
|
|---|---|---|
| Article Number | 20250051 | |
| Number of page(s) | 42 | |
| Section | Materials Science | |
| DOI | https://doi.org/10.1360/nso/20250051 | |
| Published online | 16 October 2025 | |
REVIEW
Fiber-shaped aqueous battery: Design, advancements, and perspectives
Key Laboratory of Multifunctional Nanomaterials and Smart Systems, i-Lab, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China
* Corresponding authors (emails: This email address is being protected from spambots. You need JavaScript enabled to view it.
(Ying Ling); This email address is being protected from spambots. You need JavaScript enabled to view it.
(Fan Liu); This email address is being protected from spambots. You need JavaScript enabled to view it.
(Qichong Zhang))
Received:
15
September
2025
Revised:
12
October
2025
Accepted:
14
October
2025
To meet the demand for energy storage devices with high safety, excellent flexibility, and environmental compatibility in wearable electronic devices, fiber-shaped aqueous batteries (FABs) have become a key research direction in the field of flexible energy storage. This paper systematically reviews the latest research progress of FABs. Firstly, it elaborates on their core working mechanisms, including the intercalation mechanism involving reversible insertion/extraction of charge carriers, the conversion mechanism characterized by changes in the oxidation state and phase of electrode materials and the deposition/dissolution mechanism of metal ions. Subsequently, it summarizes the design principles from three dimensions: electrode fabrication (surface coating, in-situ growth, thermal drawing, solution spinning), device architectures (parallel, twisted, coaxial, crossing), and performance evaluation metrics (energy density, specific capacity, long-term cycling stability, flexibility). Additionally, the paper combs the research breakthroughs of FABs based on Li+/Na+, multivalent ions (Zn2+/Mg2+/Ca2+/Al3+), NH4+, and alkaline systems, and introduces their applications in energy storage-photoelectric response integration, energy storage-sensing integration, and multi-device power supply. Finally, it points out the challenges, such as low utilization efficiency of electrode materials and poor interface stability, and looks forward to the development directions including intelligent materials, manufacturing technologies, and standardization construction, which provides references for the industrialization of FABs and the development of next-generation flexible energy storage technologies.
Key words: flexible energy storage / fiber-shaped aqueous batteries / intrinsic safety / multifunctional integration / wearable electronics
© The Author(s) 2025. Published by Science Press and EDP Sciences.
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The widespread proliferation of intelligent electronic devices has significantly transformed human lifestyles [1,2]. Particularly in the realm of wearable electronics, consumers are urgently demanding novel energy storage devices that integrate high safety, superior mechanical flexibility, and environmental sustainability [3]. This trend has spurred intensive research into energy storage devices possessing exceptional flexibility, tolerance to mechanical stress, and scalability for mass production [4]. Although planar flexible batteries have garnered significant attention due to their straightforward fabrication, diverse flexible conductive materials, functional gel electrolytes, ultrathin substrates, and nano-scale active materials, their practical application faces limitations in stretchability and wearing comfort [5,6]. Consequently, researchers have begun exploring the emerging technology of fiber-shaped batteries (FSBs) [7,8]. FSBs process active electrode materials into fibers, which can be woven into fabrics or textiles, exhibiting outstanding pliability and wearable characteristics [9]. Prior to 2012, research on FSBs was virtually non-existent; the pioneering work by Kim’s team [10] opened new doors for this field. With the gradual maturation of lithium-ion storage mechanisms and manufacturing strategies, novel one-dimensional (1D) FSBs have subsequently emerged, including lithium-ion batteries (LIBs) [11], lithium-sulfur (Li-S) batteries [12], metal-air batteries (MABs) [13], dual-ion batteries [14], nickel-iron batteries [15], and zinc-ion batteries [16]. All these systems, targeting high capacity, high safety, and industrial-scale production, have undergone rapid development.
Against this backdrop, fiber-shaped aqueous batteries (FABs), leveraging their unique aqueous electrolyte systems and fiber-like structural design concepts, have rapidly emerged as a highly promising research direction in the field of flexible energy storage [17–20]. Their core value lies in systematically addressing key bottlenecks in wearable applications: on one hand, the water-based ion transport mechanism fundamentally ensures the intrinsic safety and environmental benignity of the devices, completely avoiding the flammability, explosiveness and toxicity risks associated with traditional organic electrolytes; on the other hand, their fiber-shaped configuration endows them with excellent axial flexibility, radial weavability and superior adaptability to complex deformations, enabling seamless integration with textile substrates and laying the foundation for constructing truly wearable and weavable “energy fibers”. Consequently, early research primarily focused on miniaturizing and flexibilizing traditional aqueous batteries, employing carbon fibers, metal wires, and other materials as current collectors and initially achieved bendable and weavable battery structures (Figure 1A) [18,21].
 |
Figure 1 Overview of FABs. (A) Main progress in FABs. (B) Basic descriptors of common aqueous charge carriers: ionic weight (red), crystal/covalent radius (blue), and hydrated radius (green). (C) Normalized, qualitative radar comparison of each ion in terms of affordability, safety, stability, reversibility in aqueous media, and volumetric capacity. |
Benefiting from continuous material innovation and multi-scale structural engineering strategies, FABs have consistently achieved breakthroughs in key performance metrics, significantly enhancing volumetric/gravimetric energy density, rate capability, and cycling stability. The systems of FABs have expanded from the initial zinc-ion systems to diverse ones including lithium-ion, sodium-ion, aluminum-ion, nickel-iron, and nickel-bismuth (Figure 1A) [22–25]. Performance-wise, through material innovation and structural optimization, FABs have achieved high energy density, high power density, long cycle life, and outstanding mechanical stability (Figure 1B). For instance, recently reported flexible coaxial fiber-shaped aqueous zinc-ion batteries (FAZBs) with high operating voltage achieved an impressive volumetric energy density of 195.39 mWh cm−3, retaining 93.2% capacity after 3000 bending cycles (Figure 1C) [1]. Furthermore, since 2018, technologies such as metal-organic framework (MOF)-derived materials, nanostructured electrodes, and gel electrolytes have driven a performance leap in FABs. For example, MOF-derived nanoarrays (e.g., NiS2, FeS2, V2O5) grown directly on carbon nanotube fibers have significantly enhanced electrode reaction activity and cycling stability, enabling FABs to also achieve breakthroughs in high-rate charge-discharge and ultra-long cycle life (e.g., a capacity retention rate of over 74% after 20,000 cycles) [22,26–28]. These characteristics enable them not only to meet the energy demands of daily wearable devices but also to adapt to high-energy-consumption and complex environmental application scenarios. Crucially, the structural nature of FABs inherently supports functional expansion, evolving them from mere energy storage units towards intelligent system platforms. This evolution reveals immense potential for multifunctional integration, combining energy storage with sensing, display, environmental response, and more, thereby significantly reducing the integration complexity of future intelligent systems. For example, FAZBs integrated with strain sensors can simultaneously power devices and monitor human motion states in real-time.
From a broader perspective, the development of FABs provides not only a core power solution for constructing highly integrated, comfortable, and invisible smart textiles and wearable systems, driving innovation in health monitoring, human-computer interaction, and the Internet of Things (IoT), but also offers a valuable platform for fundamental scientific research. Their unique linear structure and the electrochemical processes occurring within confined spaces facilitate in-depth studies of novel energy storage materials, interface engineering, and ion transport mechanisms. Simultaneously, FABs align with the principles of green manufacturing and the circular economy. The potential use of biodegradable or recyclable materials within the aqueous system, coupled with their compatibility with continuous, scalable manufacturing processes, can significantly reduce their lifecycle environmental footprint and cost, actively supporting global carbon neutrality strategic goals. In summary, FABs by synergistically combining intrinsic safety, structural flexibility, environmental sustainability, and functional expandability, are becoming a pivotal force guiding the development of next-generation flexible energy storage technologies. They demonstrate irreplaceable strategic value in meeting current wearable device energy demands and shaping future intelligent and sustainable societies. Ongoing research will continue to focus on enhancing their comprehensive performance and integration maturity to accelerate their practical deployment.
This paper systematically reviews the latest research advances in the field of FABs. Firstly, it analyzes the key electrochemical behaviors and working mechanisms within this battery system. Building upon this, it discusses the core strategies and design principles for assembling fiber-shaped devices. Subsequently, it outlines typical fiber-shaped battery systems based on both metallic and non-metallic substrates, with a critical focus on their current developmental status. Finally, it prospects the vast application potential of such batteries in flexible electronics, leveraging their multifunctional integration capabilities.
WORKING MECHANISM OF FABS
FABs inherit the fundamental structural framework of conventional aqueous batteries, consisting of a cathode, an anode, and an electrolyte. Their energy storage behavior primarily relies on the reversible migration of charge carriers and redox reactions occurring at the electrode interfaces. The overall electrochemical performance of FABs is governed by the intrinsic physicochemical properties of the active materials, the characteristics of the charge carriers, and the underlying interaction mechanisms between them and the host structures. Consequently, a comprehensive understanding of charge storage mechanisms and the corresponding ion-material interactions is crucial for the systematic design and performance enhancement of FABs. According to the underlying electrochemical reaction pathways, these batteries can be generally classified into three types: intercalation, conversion, and deposition/dissolution mechanisms (Figure 2).
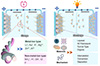 |
Figure 2 Schematic illustration of typical energy storage mechanisms in FABs. |
Intercalation mechanism
The intercalation process is the predominant energy storage method in FABs. It involves the reversible insertion and extraction of charge carriers into and from the host crystal structure without causing substantial disturbance to the framework. This mechanism applies to a variety of cations, including Li+, Na+, K+, Mg2+, Zn2+, Al3+, H+, and NH4+ [29–32]. For example, in the Prussian blue analogues’ (PBAs) structure, A+ represents the charge carrier. During discharge, A⁺ inserts into the host structure (PBA + A+ + e− → PBA-A). The process is reversed during charging. The ionic charge, hydrated radius, and specific interactions between the ions and the host matrix collectively determine the kinetics and diffusion behavior of the intercalation process [33–39]. For instance, Zn2+ typically exhibits sluggish diffusion kinetics owing to its substantial hydrated size and robust electrostatic interactions, which constrain its movement in narrow structural channels. In contrast, H+ and NH4+ can form hydrogen bonds with host structures and intercalate more easily into frameworks such as organic compounds or PBAs [40–42]. The intercalation mechanism is advantageous due to its high structural reversibility and stability, making it suitable for flexible devices with long cycle life. However, the limited number of intercalation sites constrains the achievable capacity and the rate performance is often dependent on the intrinsic conductivity and ion diffusion pathways of the electrode material.
Conversion mechanism
The conversion mechanism involves redox reactions that result in a change in the oxidation state and chemical composition of the electrode material, typically forming new phases during the charge-discharge process. Compared to intercalation, conversion reactions often involve multiple electron transfers, offering significantly higher theoretical capacities. This mechanism is prevalent in various transition metal oxides, sulfides, and nitrides (e.g., Fe3O4, Co3O4, MnO2) and is also observed in certain organic compounds featuring redox-active groups such as carbonyls or quinones [43–45]. For example, in a conversion-type material (e.g., MX, where M is metal, X is anion), A+ represents the charge carrier. During the discharge process, the material undergoes a chemical conversion (MX + A+ + e− → M + AX). The process is reversed during the charging process. In addition, some PBAs may exhibit partial conversion-like behavior, where ion insertion is accompanied by lattice rearrangement or phase transitions. The conversion mechanism is attractive for its high capacity and structural tunability; however, the associated phase changes often lead to large volume expansion, structural degradation, and unstable electrode-electrolyte interfaces, which limit cycling stability and rate performance. For fiber-based configurations, additional considerations must be made to accommodate mechanical deformation and maintain structural integrity during repeated cycling.
Deposition/dissolution mechanism
The deposition/dissolution mechanism is based on the reversible electrochemical plating and stripping of metal ions at the electrode-electrolyte interface. Typical charge carriers include Zn2+, Li+, Mg2+, and Al3+, making this mechanism suitable for metallic anode systems [46,47]. For example, using a metal M (e.g., Zn, Li, Mg, and Al) as the anode, during charging, Mn+ is reduced and deposited on the electrode (Mn+ + ne– → M). The process is reversed during discharging, where metal M dissolves in the electrolyte. Taking Zn2+ as an example, it is reduced and deposited as metallic Zn during charging and subsequently oxidized and dissolved back into the electrolyte during discharging, enabling efficient charge storage. This mechanism provides exceptionally high theoretical capacity and low operating potentials, making it highly promising for constructing high-energy-density micro-scale fiber batteries. However, challenges such as dendritic growth, uneven deposition, volumetric changes, and parasitic side reactions significantly affect cycling stability and safety. Recent strategies involving electrolyte engineering, surface modification, and current density control have shown promise in mitigating these issues, thereby advancing the applicability of this mechanism in flexible fiber-based devices.
DESIGN PRINCIPLES OF FABS
To achieve high-performance, wearable and integrable energy storage capabilities, the design of FABs requires a holistic consideration of material fabrication, structural configuration, and high electrochemical output. The choice of electrode fabrication techniques, the architectural design of the device, and the selection of performance evaluation metrics all play critical roles in determining the overall efficiency and practical applicability of these systems. Therefore, a systematic summary of the design principles of FABs is essential for guiding the coordinated optimization of materials and devices. In the following sections, we provide a detailed overview from the perspectives of electrode fabrication methods, device architectures, and key performance metrics.
Electrode fabrication techniques
In FABs, electrodes not only serve as pathways for electron transport but also act as structural platforms for hosting active materials, similar in function to current collectors in conventional battery systems. However, due to the one-dimensional geometry and limited surface area of fiber substrates, achieving uniform and stable loading of active materials on their surfaces presents significant challenges compared to planar configurations, particularly when flexibility and structural integrity must be preserved. These constraints place greater demands on electrode fabrication techniques, requiring high precision, compatibility, and scalability. To date, commonly employed strategies for constructing fiber electrodes include surface coating, in situ growth, wet spinning, and thermal drawing, each offering distinct advantages in terms of material integration and device adaptability. The following subsections provide a comprehensive overview and comparative analysis of these fabrication approaches.
In-situ growth
In-situ growth is a widely adopted strategy for constructing fiber electrodes with enhanced interfacial contact, mechanical robustness, and structural integration [48,49]. Unlike surface coating, where pre-synthesized materials are externally applied, in-situ growth involves the direct formation or crystallization of active materials on the fiber substrate, typically through methods such as electrodeposition, hydrothermal/solvothermal synthesis, and chemical bath deposition (Figure 3A). Electrodeposition enables the conformal growth of conductive or electrochemically active layers on fiber current collectors with precise control over thickness, morphology, and composition. Hydrothermal and solvothermal techniques are particularly suitable for the synthesis of crystalline transition metal compounds (e.g., oxides, hydroxides, PBAs) with strong substrate adhesion. These methods not only improve the utilization of active materials but also enhance the mechanical stability of the fiber electrode under repeated deformation. However, in-situ growth methods often involve strict reaction conditions (e.g., temperature, pH, time) and may be limited in terms of substrate compatibility or large-scale uniformity. Moreover, controlling the growth orientation and achieving homogeneous deposition along long fiber lengths remain technical challenges. Nonetheless, the in-situ strategy is considered highly promising for developing integrated and high-performance fiber electrodes, particularly for wearable and flexible electrochemical devices.
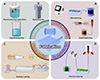 |
Figure 3 Schematic illustration of typical fabrication methods for fiber electrodes. (A) In situ growth. (B) Solution spinning. (C) Surface coating. (D) Thermal drawing. |
Solution spinning
Solution spinning, encompassing wet spinning and electrospinning, is a versatile approach for constructing fiber electrodes by integrating active materials into or onto fiber substrates (Figure 3B) [50,51]. This strategy allows for the controlled formation of flexible, porous, and electrochemically active structures, which are particularly advantageous for wearable energy storage devices. In wet spinning, a homogeneous solution or dispersion containing active materials and spinnable polymers is extruded into a coagulation bath, where fiber formation occurs via phase separation. The resulting composite fibers can serve directly as self-supporting electrodes or be woven into textile-based energy systems. In contrast, electrospinning is primarily employed to deposit a nanofiber layer containing electrochemically active components onto the surface of a conductive fiber substrate. Driven by a high-voltage electric field, a polymer or sol-gel solution is stretched into continuous nanofibers and deposited directly onto the fiber surface, forming a porous and conformal coating. This method significantly increases the effective surface area and improves interfacial contact between the active material and the fiber core. Despite their advantages, solution-spun structures often suffer from limited conductivity and insufficient mass loading, particularly when a large proportion of insulating polymer is used. Therefore, post-treatment strategies such as carbonization, ion crosslinking, and secondary coating are commonly employed to enhance their mechanical strength and electrochemical performance.
Surface coating
Surface coating is one of the most straightforward and widely employed techniques for fabricating fiber electrodes, owing to its simplicity, scalability, and broad material compatibility [52,53]. As shown in Figure 3C, pre-synthesized active materials are deposited onto fiber substrates through methods such as dip-coating, spray-coating, and drop-casting. These techniques enable the formation of uniform active layers on the fiber surface, providing accessible electrochemical interfaces and facilitating efficient ion and electron transport. Despite its simplicity, surface coating faces several challenges specific to the one-dimensional geometry of fibers. Compared to planar substrates, fiber surfaces possess a smaller contact area and higher curvature, which can hinder the uniform adhesion of active materials and result in poor mechanical robustness or interfacial delamination during cycling. Furthermore, coated layers may crack, peel, and suffer from limited mass loading due to weak interaction with the fiber core, especially under repeated mechanical deformation in wearable applications. To address these issues, recent studies have explored strategies such as using intermediate adhesion layers, optimizing coating viscosity and surface energy, and introducing conductive binders and nanostructures (e.g., carbon nanotubes (CNTs), graphene) to enhance interfacial bonding and electrical connectivity. Surface coating remains a highly versatile method, particularly suitable for the rapid screening of active materials and the scalable integration of hybrid or multifunctional components onto fiber substrates.
Thermal drawing
Thermal drawing is an emerging and highly integrative fabrication technique for constructing fiber-shaped energy storage devices [54,55]. Originally developed for optical fiber production, this method has been adapted to embed multiple functional components, such as electrodes, electrolytes, and current collectors, into a single continuous fiber via a thermal drawing process. In a typical process, a macroscopic preform is fabricated with a well-defined multilayer structure composed of thermally compatible materials. Upon heating above the softening temperature of the polymer cladding, the preform is continuously drawn into meters-long microstructured fibers, preserving the original cross-sectional architecture at a reduced scale. This enables the co-integration of electrode conductors, solid and gel electrolytes, and encapsulating layers within a unified fiber body. Importantly, thermal drawing provides a powerful platform for the continuous and scalable production of FSBs, making it especially attractive for practical applications in textile-based energy systems. It offers precise control over fiber geometry, material distribution, and mechanical uniformity, enabling mass production with excellent repeatability. However, the technique is limited by the thermal compatibility of active components with the drawing temperature, as well as the requirement for polymer matrices that can encapsulate electrochemical materials without compromising performance. Moreover, improving the loading of active materials and enhancing ionic/electronic conductivity within the drawn fibers remains a key challenge.
To provide a clearer comparison among different electrode fabrication techniques, Table 1 summarizes the key technical distinctions, highlighting their respective advantages and limitations in terms of scalability, cost, and performance optimization potential.
Summary of electrode fabrication techniques
Device architectures
In FABs, in addition to electrode materials and fabrication techniques, device architecture also plays a pivotal role in determining overall performance. The structural configuration not only governs the transport pathways of ions and electrons but also significantly affects interfacial contact area, mechanical flexibility, and the compatibility with wearable systems. Depending on the assembly strategy, fiber batteries can be constructed in several typical configurations, each offering distinct advantages in terms of physical structure and electrochemical behavior. Representative architectures include: (i) parallel structures, where two fiber electrodes are aligned side-by-side to form a basic energy storage unit; (ii) twisted structures, in which the electrodes are helically intertwined to enhance interfacial contact and mechanical stability; (iii) coaxial structures, where the cathode, electrolyte and anode are concentrically aligned to improve spatial utilization and charge transport efficiency; (iv) crossed structures, where perpendicularly arranged electrodes form grid-like arrays, offering improved adaptability for textile integration. A comprehensive understanding of these structural configurations and their relationship with device performance is essential for the rational design and application-specific optimization of FABs.
Parallel structure
As shown in Figure 4A, the parallel configuration is one of the most fundamental and widely applied device architectures in FABs. In this configuration, two fiber electrodes are arranged side-by-side and encapsulated within an electrolyte layer, without direct physical contact. Owing to its structural simplicity and ease of assembly, the parallel layout has been extensively used in various FABs. This configuration is particularly advantageous for scalable fabrication, as it requires minimal alignment precision and is compatible with roll-to-roll, knitting, and weaving processes. Moreover, the physical separation of the two electrodes allows for independent material design and reduces the risk of internal mechanical failure. However, the absence of mechanical coupling between electrodes also introduces challenges. In liquid electrolytes, the two electrodes may move and come into contact under deformation or external perturbations, leading to short-circuit risks. In contrast, twisted configurations can stabilize electrodes through mechanical entanglement. To mitigate this issue, semisolid or solid-state electrolytes with adhesive or encapsulating functions have been employed to maintain inter-electrode spacing and improve device safety. Overall, the parallel configuration offers an effective pathway for cost-efficient and scalable manufacturing of fiber-shaped batteries, particularly in scenarios where mechanical strain is minimal and device simplicity is prioritized.
 |
Figure 4 Schematic illustrations of typical device architectures for FABs. (A) Parallel structure. (B) Twisted structure. (C) Coaxial structure. (D) Crossing structure. |
Twisted structure
The twisted configuration refers to a structure where two individual fiber electrodes are helically wound together with a defined pitch and angle (Figure 4B). This configuration forms a mechanically entangled structure that enhances flexibility and facilitates repeated deformation, making it particularly suitable for wearable and stretchable applications. Compared with coaxial structures, twisted fiber devices typically exhibit a smaller effective contact area between the electrodes, which may limit interfacial charge transfer. To compensate for this, it is essential to coat active materials on both fiber electrodes prior to assembly, ensuring sufficient electrochemical activity. Nonetheless, the twisted configuration offers distinct advantages in fabrication: it simplifies the construction process of multi-material fiber devices, avoids the complexities of sequential layer deposition on curved surfaces and enables higher production throughput. For example, in fiber-based solar cells or supercapacitors, it is often more feasible to twist together two pre-treated fibers than to build coaxial structures with uniform concentric coatings. The helical nature of the twisted configuration introduces multiple localized contact points between the electrodes, promoting effective mass and charge transport. The geometry of the twist configuration, particularly the pitch and angle, plays a crucial role in determining the electrochemical performance. A tighter twist generally enhances interfacial contact, thus improving charge transfer efficiency, while an overly tight configuration may introduce mechanical strain or short-circuit risk. Therefore, optimizing the twisting parameters is key to balancing performance, reliability, and mechanical robustness. Due to its structural simplicity, scalability, and compatibility with textile integration techniques, the twisted configuration has been widely applied in various fiber-based energy devices, including supercapacitors, solar cells, and batteries.
Coaxial structure
As shown in Figure 4C, the coaxial configuration features a concentric core-shell structure in which the electrode, electrolyte, and counter electrode are sequentially deposited along the radial direction of a fiber substrate. This geometry allows for continuous interfaces between layers and a highly compact architecture, making it especially appealing for FABs where efficient ion transport, mechanical stability, and integration density are essential. In this design, a fiber electrode, typically serving as either the anode or cathode, is first prepared as the central core, followed by conformal coating of the electrolyte and the outer electrode layer. The close physical contact between each layer facilitates rapid charge and mass transport, minimizes internal resistance, and enhances overall electrochemical kinetics. Moreover, the coaxial layout effectively protects the internal layers from mechanical damage and environmental exposure, improving long-term durability and device reliability. The coaxial structure draws inspiration from conventional layered planar batteries but adapts these features to the cylindrical geometry of fibers. To ensure stable operation under deformation, each functional layer must deform coherently with the others, which requires mechanical compatibility and uniform thickness across the entire device length. Maintaining a stable interface during bending, twisting, and stretching remains a major consideration. A variety of fabrication techniques, including dip-coating, extrusion, and 3D printing, have been employed to realize coaxial fiber batteries. However, these processes face considerable challenges when applied to curved fiber surfaces, particularly in achieving thin, uniform, and defect-free coatings over long distances. Incompatibility of material properties (e.g., viscosity mismatch or poor interfacial adhesion) may result in delamination or local failure, hindering performance and scalability. Despite these limitations, the coaxial configuration remains one of the most promising structural strategies for developing FABs with high energy efficiency, robust mechanical performance, and good environmental tolerance.
Crossing structure
The crossing structure is a grid-like architecture where multiple fiber electrodes are arranged orthogonally through textile-compatible methods such as weaving or embroidery. As shown in Figure 4D, in this structure, each intersection point between a cathode and an anode fiber can serve as an independent electrochemical cell, enabling the formation of multi-node fiber battery arrays within a single fabric. This configuration is particularly attractive for the integration of energy storage systems into electronic textiles. It allows for high integration density, spatial programmability, and modular energy supply design. By controlling the number and layout of electrode intersections, the total output voltage and capacity of the system can be tailored to specific application needs, offering a scalable route to power distributed or multi-functional wearable electronics. Moreover, the discrete nature of electrochemical units at fiber intersections offers advantages in fault tolerance. Even if a single unit fails, the rest of the battery network can continue to function, improving system-level reliability. Although the crossing structure has been conceptually demonstrated in several types of fiber-based electronic devices, such as fiber photodetectors, organic electrochemical transistors, there remain few studies that specifically implement and systematically investigate this structure in FABs [56–59]. This scarcity may be attributed to the technical challenges associated with ensuring reliable electrochemical isolation and mechanical robustness at the intersection points. Nevertheless, the successful demonstrations of crossing architectures in other fiber-optoelectronic systems suggest that similar design principles could be extended to FABs, particularly for scalable textile integration and distributed energy supply. However, the performance of crossed fiber batteries relies heavily on the electrical contact quality at each junction. The absence of continuous electrolyte encapsulation along the fiber length may result in increased contact resistance or uneven ion diffusion at the intersection points. To overcome this, gel or solid-state electrolytes are often used to ensure stable and localized ionic pathways and mechanical coupling. Overall, the crossed configuration holds great promise for realizing flexible, integrated and addressable power sources in smart textiles.
In summary, structural configuration plays a vital role in determining the electrochemical performance, mechanical flexibility, and integration potential of FABs. Parallel and twisted configurations are widely adopted due to their simplicity and compatibility with scalable fabrication. Coaxial structures, although more complex to manufacture, offer superior charge transport, space utilization, and environmental stability. Crossed architectures, on the other hand, enable modular energy distribution and integration into textile systems with high addressability. Each configuration exhibits distinct trade-offs between fabrication difficulty, performance optimization, and application scenarios. Moving forward, rational selection or hybrid integration of these structures, guided by device function and usage conditions, will be key to advancing high-performance, multifunctional fiber-based energy storage systems. In particular, future efforts should focus on scalable fabrication strategies, robust interface engineering, and mechanical adaptability across configurations to fully unleash the potential of fiber battery technologies.
Performance evaluation metrics
To assess the practical applicability and performance potential of FABs, a comprehensive evaluation framework encompassing both electrochemical and mechanical metrics is essential. In contrast to conventional planar devices, FABs are defined by their 1D structure, mechanical flexibility, and integration capability with wearable electronics, all of which present unique challenges for performance evaluation. Core metrics include energy density and specific capacity, which reflect the device’s energy storage capability; long-term cycling stability, which determines its operational durability under repeated use; and mechanical flexibility, which governs its ability to function reliably under dynamic deformation. A systematic evaluation of these parameters is critical not only for benchmarking new materials and device architectures but also for guiding future development toward application-oriented, scalable, and durable fiber-based energy systems.
Energy density
Energy density is a primary metric that reflects the energy storage capability of FABs, which is typically reported in terms of Wh g−1, Wh cm−2, and Wh cm−3. Gravimetric energy density evaluates the energy output relative to the mass of active materials, making it essential for material-level comparisons, especially with conventional planar batteries. In contrast, volumetric energy density considers the entire device volume and is particularly relevant for applications where space efficiency is critical, such as in integrated wearable or implantable electronics. Areal energy density is also frequently reported, especially in studies where the fiber is assembled into planar configurations or when cross-sectional dimensions are well defined, offering an additional perspective for performance comparison. Achieving high energy density in FABs requires the synergistic design of high-capacity electrode materials, compact and efficient architectures (e.g., coaxial, twisted and core-sheath configurations) and high-conductivity electrolytes that enhance ion transport and active material utilization. Given the growing demand for lightweight, miniaturized, and wearable power sources, both gravimetric and volumetric energy densities remain critical indicators for evaluating and comparing FABs’ performance.
Specific capacity
Specific capacity, typically expressed in mAh g−1, mAh cm−2, and mAh cm−3 is the fundamental electrochemical parameter that quantifies the amount of electric charge stored per unit mass of active material. It directly reflects the intrinsic charge-storage capability of the electrode materials and serves as a baseline for evaluating the overall energy density and power output of FABs. Accurate determination of specific capacity requires normalization by the mass of active components only, excluding contributions from current collectors, substrates, and encapsulation layers, to ensure meaningful comparisons between different material systems and device architectures. In the context of FABs, achieving high specific capacity is particularly challenging due to constraints on mass loading and geometric limitations inherent to fiber-based configurations. Therefore, the development of advanced electrode materials with high theoretical capacities, rapid redox kinetics, and structural stability under deformation is essential. Furthermore, the fiber geometry often necessitates innovative fabrication strategies to increase the effective utilization of active materials, such as hierarchical nanoarchitectures, porous structures, and uniform coatings on high-surface-area conductive cores. Improvements in specific capacity not only enhance the energy storage capability but also contribute to extending the cycle life and rate performance, which are crucial for the deployment of FABs in practical, high-demand wearable applications.
Long-term cycling stability
Long-term cycling stability is a critical parameter for assessing the operational reliability and lifespan of FABs under repeated charge-discharge conditions. It is typically evaluated by measuring the capacity retention over hundreds to thousands of cycles, often under varying current densities and mechanical states. High cycling stability is especially important for wearable electronics, where batteries may be subjected to frequent electrochemical cycling, environmental exposure, and mechanical deformation. Capacity degradation in FABs can arise from multiple sources, including irreversible structural changes in the electrode materials, dissolution or detachment of active components, degradation of the electrolyte, and deterioration of interfacial contact. To mitigate these issues, advanced design strategies such as using chemically stable and mechanically robust electrode materials, introducing protective layers to suppress material dissolution and engineering flexible current collectors with high adhesion strength are often employed. Furthermore, integrating self-healing polymers or hydrogel electrolytes can buffer mechanical damage and maintain interface stability, thereby prolonging cycling life. A high-capacity retention rate after long-term cycling is a hallmark of a reliable and durable fiber-shaped energy storage system.
Flexibility
Flexibility is a defining feature of FABs, enabling their seamless integration into deformable, wearable, and textile-based systems. A flexible battery must maintain stable electrochemical performance under various mechanical deformations such as bending, twisting, stretching, and compression. Flexibility is typically evaluated by monitoring capacity retention, resistance change, and voltage stability under cyclic mechanical loading, as well as under dynamic usage conditions that simulate real-world applications. Achieving high flexibility without compromising electrochemical performance requires a holistic materials-to-device approach. This includes the use of intrinsically flexible substrates and current collectors (e.g., carbon nanotube fibers, conductive polymers, and metallic yarns), mechanically compliant electrode materials, and stretchable or gel-based electrolytes. Structural engineering, such as coaxial, twisted, and crossing structure, also plays a key role in stress distribution and mechanical resilience. In addition, ensuring strong interfacial adhesion between components is critical for avoiding delamination or contact failure during repeated deformation. Ultimately, the ability of FABs to retain functionality under mechanical stress is fundamental to their real-world deployment in next-generation wearable and smart electronic systems.
RESEARCH PROGRESS ON FABS
Fiber-shaped aqueous lithium-ion and sodium-ion batteries
With the rapid development of wearable electronics, smart textiles, and flexible sensors, energy storage devices are required to simultaneously deliver light weight, safety, bendability, and weavability [60,61]. Conventional lithium-ion batteries based on organic electrolytes, although offering high energy density, suffer from flammability and leakage, making them unsuitable for flexible electronics where safety is paramount. In contrast, aqueous electrolytes are intrinsically safe, cost-effective and environmentally benign [62,63]. When further combined with fiber-shaped architectures, batteries can be seamlessly integrated with textiles, representing an ideal format for flexible energy systems. Against this backdrop, fiber-shaped aqueous lithium-ion batteries (FALBs) have emerged, coupling the high energy density of lithium-ion chemistry with the intrinsic safety of aqueous electrolytes, thus offering a promising power source for flexible electronics and smart textiles.
In 2016, Zhang et al. [17] fabricated a FALB using polyimide/CNT hybrid fiber anodes and LiMn2O4/CNT hybrid fiber cathodes (Figure 5A). The device delivered a remarkable power density of 10217.74 W kg−1 and an energy density of 48.93 Wh kg−1 (Figure 5B), comparable to thin-film lithium-ion batteries, while fundamentally eliminating the safety hazards associated with flammable organic electrolytes. Moreover, the inherent weavability of fiber-shaped architectures further broadened their application scenarios in flexible energy textiles (Figure 5C). Subsequently, Dai et al. [64] proposed a fluorine-free, high-voltage dual-cosolvent electrolyte, which expanded the electrochemical stability window to 3.3 V and enabled excellent electrochemical performance in FALBs, highlighting the potential of aqueous electrolytes for high-voltage energy storage devices (Figure 5D).
 |
Figure 5 Research progress of lithium-ion and sodium-ion batteries. (A) Schematic illustration of a simplified structure of the FALBs. (B) Energy and power densities of the FALBs compared with previous energy storage systems. (C) Energy textile woven with FALBs. (A–C) Reproduced with permission from [17]. Copyright©2016, Royal Society of Chemistry. (D) Schematic diagram of the high-voltage flexible aqueous fiber LIB based on synergistic dual co-solvents hybrid electrolyte. Reproduced with permission from [64]. Copyright©2024, Elsevier. (E) Schematic illustration of the structure of FASBs. (F) Galvanostatic charge-discharge curves of FASBs. (G) Capacity stability of FASBs. (E–G) Reproduced with permission from [65]. Copyright©2017, Elsevier. (H) Schematic and charge-discharge curves of FASBs. Reproduced with permission from [24]. Copyright©2019, The Author(s). |
Nevertheless, the limited reserves, uneven distribution, and rising cost of lithium resources are gradually becoming bottlenecks hindering the widespread deployment of FALBs. This has shifted attention toward sodium-based systems, which share similar chemical properties with lithium but benefit from abundant reserves, low cost, and strong sustainability, making them highly attractive for large-scale storage and wearable electronics. Motivated by this, fiber-shaped aqueous sodium-ion batteries (FASBs) have rapidly emerged, integrating sodium-based electrodes with flexible fiber architectures to inherit the intrinsic safety of aqueous systems while alleviating the resource and cost constraints of lithium.
Guo et al. [65] employed Na0.44MnO2 as the cathode and carbon-coated NaTi2(PO4)3 as the anode to construct a FASB (Figure 5E). Operating in 1 M Na2SO4 electrolyte within a 0–1.6 V voltage window, the device delivered a discharge capacity of 46 mAh g−1 at 0.1 A g−1, retained 12 mAh g−1 even at 3 A g−1 and maintained 76% of its capacity after 100 cycles (Figure 5F, G), demonstrating favorable rate capability and cycling stability. Further, He et al. [24] developed a facile in situ growth strategy to synthesize KNiFe(CN)6 nanotube cathodes and NaTi2(PO4)3 anodes directly on CNT fiber substrates, assembling the first high-performance quasi-solid-state FASBs (Figure 5H). The device delivered a high volumetric capacity of 34.21 mAh cm−3 and a volumetric energy density of 39.32 mWh cm−3, while maintaining outstanding mechanical flexibility. This binder-free fiber electrode design provides a new technological pathway for wearable energy systems and flexible power textiles.
In summary, fiber-shaped aqueous ion batteries combine safety, flexibility, and wearability, showing broad prospects in the field of flexible energy storage. FALBs offer a balance of high energy density and intrinsic safety, while FASBs demonstrate stronger sustainability owing to their resource abundance and low cost. Nevertheless, challenges remain in electrode structural stability, electrolyte compatibility, and performance retention under multi-stress conditions. Therefore, future efforts should focus on the design of stable electrode materials, electrolyte engineering, and seamless integration of fiber batteries into textiles to advance their practical applications in smart wearable electronics.
Fiber-shaped aqueous zinc-ion batteries
FAZBs have attracted significant attention owing to their unique advantages [66,67]. Zinc offers several intrinsic merits, including a small ionic radius (0.74 Å), a low redox potential (Zn2+/Zn: −0.76 V) and a high theoretical volumetric capacity (5855 mAh cm−3), surpassing lithium- and sodium-based systems in volumetric energy storage capability [68–70]. From a practical perspective, Zn exhibits high reversibility in aqueous environments, greatly reducing risks of combustion or explosion, while its abundance and low cost further support scalable applications. In FAZBs, Zn2+ reversibly migrates between the electrolyte and cathode, undergoing intercalation/deintercalation, sometimes coupled with H+-driven phase transitions, to achieve reversible energy storage [71]. Key challenges, however, include cathode dissolution-redeposition and structural degradation, Zn dendrite growth and parasitic reactions at the anode, as well as interface delamination and ion transport limitations under bending conditions. Currently, cathode materials are dominated by Mn-based compounds [72–74], V-based oxides [73,75,76], and PBAs [77–79], each critically determining device performance.
Mn-based materials are the most widely used cathodes in FAZBs due to their low cost and high capacity. In an early demonstration (2013), Yu et al. [80] reported a flexible fiber-type Zn-C battery using commercial carbon fibers as the current collector, MnO2/graphite as the cathode, a Zn wire anode, and a ZnCl2/NH4Cl electrolyte (Figure 6A). The device delivered a discharge capacity of 158 mAh g−1 (Figure 6B) and was able to power a light-emitting diode (LED) (Figure 6C), though it was non-rechargeable and offered limited capacity. Later, Li et al. [81] realized a rechargeable yarn zinc-ion battery (ZIB) by integrating α-MnO2 cathodes with Zn anodes into coaxial helical yarn electrodes and a crosslinked polyacrylamide (PAM) gel electrolyte. Owing to its high ionic conductivity and yarn architecture, the device delivered high volumetric energy density, stable cycling, and excellent weavability/stretchability, successfully powering a light-emitting textile panel (Figure 6D). Mn-based materials offer versatile adaptability across electrolytes and conditions, yet suffer from Mn dissolution and phase degradation caused by Zn2+/H+ co-intercalation and structural reconstruction. Boosting both energy density and lifetime remains the key challenge for Mn-based FAZBs.
 |
Figure 6 Research progress of zinc-ion batteries. (A) The schematic illustration of the fiber battery based on Zn wire and MnO2/carbon fiber. (B) The discharge curves of fiber battery with Zn wire or Zn powder/carbon fiber as negative electrode. (C) Two fiber batteries connected in series continuously drive a commercial green LED when bent around human finger. (A–C) Reproduced with permission from [80]. Copyright©2013, Elsevier. (D) Schematic of fabrication and encapsulation of yarn ZIBs to power luminescent panel. Reproduced with permission from [81]. Copyright©2018, American Chemical Society. (E) Schematic illustration of quenched Ni-V2O5 NWs@CNT fiber electrode. (F) Galvanostatic charge-discharge (GCD) curves. (E, F) Reproduced with permission from [82]. Copyright©2012, Elsevier. (G) Diagram of synthesis procedure of a-Ca-V2O5/CNTF. (H) Comparison of rate performance. (G, H) Reproduced with permission from [83]. Copyright©2023, Wiley-VCH. (I) Schematic of flexible high-voltage coaxial of FAZBs. Reproduced with permission from [1]. Copyright©2019, American Chemical Society. (J) Schematic illustration of as-fabricated FAZIB based on dual-layer gel electrolyte with lysine. (K) Cyclic property and coulombic efficiency of as-assembled FAZIB. Inset: GCD splines at selected cycle. (J, K) Reproduced with permission from [27]. Copyright©2024, Wiley-VCH. |
V-based cathodes provide an alternative with simpler storage mechanisms and rapid ion transport, owing to the multiple valence states of V and the structural flexibility of V-O polyhedra. Various V-based oxides, including V2O5, VO2, and V2O3, have been investigated, with V2O5 being the most studied. Its layered structure, composed of corner- and edge-sharing VO5 pyramids with a large interlayer spacing of 5.8 Å, offers ideal channels for reversible Zn2+ intercalation. Li et al. [82] employed millisecond quenching to engineer defects, enabling metal-ion doping and oxygen vacancies to synergistically tailor the local electronic environment of Ni-V2O5 nanowires. This strategy enhanced charge transfer and introduced abundant Zn2+ storage sites, yielding a quasi-solid-state fiber-shaped zinc-ion battery (FAZIB) with a high volumetric energy density of 90.3 mWh cm−3 when paired with a Zn NSs@CNT anode (Figure 6E, F). The device was further integrated into magnetically actuated fiber soft robots, showcasing combined energy storage and actuation capability. Similarly, Guo et al. [83] synthesized amorphous Ca-V2O5 nanostructures via in situ electrochemical oxidation of Ca-doped VO2 arrays (Figure 6G). The amorphization effectively activated abundant Zn storage sites, achieving high volumetric capacity and rapid reaction kinetics for excellent rate capability (Figure 6H). Despite these advantages, V-based materials still face challenges such as low operating potential and interfacial side reactions. Future work may focus on structural optimization, interfacial stabilization, and the design of novel vanadium-based compounds to advance their practical deployment.
PBAs stand out for their open three-dimensional framework, relatively high working voltage (1.5–1.8 V), low toxicity, and cost-effectiveness. They are compatible with fiber current collectors and low-temperature deposition/coating processes, making them highly suitable for high-voltage fiber devices. To address the rising energy demands of textile-based wearable electronics, Zhang et al. [1] developed the first coaxial-fiber aqueous rechargeable zinc-ion battery (CARZIB) using spherical ZnHCF as the cathode. The device achieved a high volumetric capacity of 100.2 mAh cm−3 and an energy density of 195.39 mWh cm−3, while maintaining excellent flexibility, retaining 93.2% of capacity after 3000 bending cycles (Figure 6I). When woven into textiles, CARZIBs delivered both high voltage and high current outputs, sufficient for powering high-consumption devices. However, severe Zn dendrite formation during stripping and electrode-electrolyte delamination under folding remains major obstacles. To overcome these issues, Li et al. [27] developed an advanced dual-layer gel electrolyte system, which comprises a poly(vinyl alcohol)/zinc acetate (PVA/Zn(OAc)2) inner gel and a mechanically robust zinc-alginate outer layer. Furthermore, lysine was introduced as an additive to facilitate the formation of a stable solid electrolyte interphase (SEI) on the zinc anode surface. The resulting Zn/ZnHCF fiber battery demonstrated outstanding mechanical durability, retaining 97.7% of capacity after 500 bending cycles (Figure 6J, K). This dual-gel electrolyte design establishes a promising foundation for the development of long-life FAZBs.
In summary, FAZBs have achieved systematic progress across materials, architectures, electrolytes, and interfacial engineering. With the convergence of precise material design and scalable fiber-fabrication technologies, FAZBs are poised to transition from proof-of-concept demonstrations to engineered solutions for high-power wearable and textile-integrated energy systems [84,85].
Fiber-shaped aqueous magnesium-ion, calcium-ion, and aluminum-ion batteries
Multivalent-ion storage systems, leveraging multi-electron transfer processes (Mg2+/Ca2+: 2e−; Al3+: 3e−), offer significantly enhanced volumetric capacity at equivalent redox potentials, resulting in intrinsically high theoretical capacities and energy densities [86,87]. Meanwhile, the natural abundance of magnesium, calcium, and aluminum in the Earth’s crust (Mg ≈ 1.94 wt%, Ca ≈ 3.6 wt%, Al ≈ 8.23 wt%) ensures wide availability, low cost, and strong sustainability [88–90]. Coupled with the intrinsic safety, high ionic conductivity, and environmental benignity of aqueous electrolytes, fiber-shaped multivalent aqueous batteries emerge as promising candidates for flexible, weavable, and integrable power supplies tailored to smart textiles, wearable electronics, and soft sensing systems.
In the Mg2+ system, He et al. [91] first showed that layered NiOOH, long used in alkaline batteries, can reversibly host Mg2+ in neutral aqueous electrolytes via a proton-assisted mechanism, exhibiting a discharge plateau at 0.57 V (Figure 7A). Building on self-supporting fibrous designs, they assembled a NaTi2(PO4)3//NiOOH “rocking-chair” fiber-shaped aqueous Mg-ion batteries (FAMBs), which simultaneously achieved high energy density and excellent mechanical flexibility, enabling seamless weaving into textiles for powering optoelectronic devices (Figure 7B, C). Ling et al. [92] further employed organic-acid-assisted coordination and etching to construct defect-rich, K-free, water-containing CuHCF (D-CuHCF@CNTF) arrays on CNT fibers (CNTF). Exploiting high-valence active sites and ordered array architecture, the cathode delivered a reversible capacity of 146.6 mAh g−1, approaching the theoretical two-electron limit (Figure 7D). When paired with a NaTi2(PO4)3/CNTF anode, the resulting FAMBs demonstrated both high energy density and mechanical compliance (Figure 7E, F).
 |
Figure 7 Research progress of magnesium-ion, calcium-ion and aluminum-ion batteries. (A) GCD curves at 8 A g−1 of NiOOH/CNT@CC obtained in 1 M MgCl2 and 1 M KOH. (B) Schematic diagram of the as-assembled FAMIBs. (C) Optical photo and structural schematic of the self-powered photoelectric sensing fabric. (A–C) Reproduced with permission from [91]. Copyright©2024, The Author(s). (D) The comparison of GCD curves from three electrode. (E) Schematic diagram of the FAMIBs. (F) Capacity retention of FAMIBs under different bending angles. (D–F) Reproduced with permission from [92]. Copyright©2024, The Author(s). (G) Schematic illustration of the full configuration of FACIBs. (H) GCD profiles under different bending angles at a fixed length (2 cm) and bending radius (0.5 cm) (inset: photographs for the bending test). (G, H) Reproduced with permission from [93]. Copyright©2024, Elsevier. (I) GCD profiles at different bending angles of fiber aqueous Al-ion battery. Reproduced with permission from [25]. Copyright©2022, The Author(s). |
For Ca-ion systems, Liu et al. [93] developed a self-supporting ZnHCF@CF cathode, where Ca2+/H+ co-intercalation enabled efficient storage within an expanded voltage window. The assembled ZnHCF@CF//PANI@CF fiber-shaped aqueous Ca-ion batteries (FACBs) achieved a volumetric energy density of 43.2 mWh cm−3, while maintaining stable performance under multiple deformation modes, underscoring their potential in wearable applications (Figure 7G, H). In the Al-ion domain, Xiong et al. [25] reported a stretchable fiber-shaped aqueous Al-ion battery consisting of a manganese hexacyanoferrate cathode, a graphene oxide-modified MoO3 anode, and a hydrogel electrolyte. The device retained 91.6% capacity after 100 cycles at 1 A cm−3, delivering a capacity of 42 mAh cm−3 and an energy density of 30.6 mWh cm−3. Integrated seamlessly into textiles, it reliably powered LEDs, validating its application in stretchable and wearable power systems (Figure 7I).
Fiber-shaped multivalent-ion aqueous batteries hold strong potential for flexible energy storage but face intrinsic hurdles, including sluggish ion transport from large hydrated cations, narrow electrolyte stability windows, and trade-offs between active loading and mechanical flexibility. Recent advances are beginning to address these limitations through defect and interlayer engineering of electrodes, electrolyte innovations such as water-in-salt and hydrated eutectics and optimized fiber architectures that enhance capacity while retaining robustness. Looking forward, the integration of energy-harvesting and functional fibers promises the development of self-sustaining, weavable smart-textile power systems. In summary, Mg-, Ca-, and Al-based FABs, with their sustainability, high theoretical capacities, and compatibility with flexible form factors, are emerging as strong candidates for next-generation wearable energy storage. Future research should emphasize synergistic optimization of electrodes and electrolytes, as well as system-level integration of device architectures and multifunctionality, to unlock comprehensive improvements in performance, stability, and real-world applicability.
Fiber-shaped aqueous ammonium-ion batteries
Among various fiber-shaped energy storage technologies, fiber-shaped aqueous ammonium-ion batteries (FAABs) have recently emerged as a promising alternative due to their unique charge carriers, fast ion kinetics and intrinsic safety. Distinct from traditional metal-ion systems, the utilization of NH4+ ions offers advantages such as small hydrated ionic radius, high mobility in aqueous electrolytes and low redox potential, enabling rapid charge transport and high power density. These features, combined with the structural advantages of fiber-shaped architectures, such as flexibility, mechanical robustness and textile integration, create new opportunities for the development of next-generation wearable energy storage devices. In this section, we will focus on recent progress in FAIBs, highlighting material design strategies, device configurations and electrochemical performance.
Li et al. [94] demonstrated a novel FAIBs featuring high rate capability and outstanding cycling stability. As illustrated in Figure 8A, B, the device employed urchin-like NH4V4O10 nanostructures coated onto carbon fiber as the cathode and polyaniline (PANI) nanorods grown on carbon fiber as the anode, operating in an (NH4)2SO4 aqueous electrolyte. Benefiting from the fast diffusion kinetics of NH4+, the battery delivered a high specific capacity of 167 mAh g−1 at 0.1 A g−1, retained 54 mAh g−1 even at 1 A g−1, and exhibited an ultralong cycle life with excellent capacity retention. These results highlight the great potential of FAABs for high-performance fiber-shaped energy storage in wearable electronics. However, despite the promising performance, the relatively low capacity and limited mechanical flexibility of existing NH4+ storage materials still pose significant challenges for practical FAABs integration. Among them, PBAs exhibit high operating plateaus but often suffer from poor structural stability during cycling, which seriously hampers their practical application. To address these limitations, Han et al. [95] designed a Zn-doped dual-active-site copper hexacyanoferrate (ZnCuHCF) as a high-energy and ultrastable cathode material for NH4+ storage. Via an innovative in-situ dynamic compensation strategy to prepare Zn-doping dual-active-site CuHCF cathode (Figure 8C), where Zn2+ doping suppresses Jahn-Teller distortions via orbital fission and stabilizes the lattice during NH4+ intercalation. The incorporation of Zn into the structure initiates a transition in the Cu–N coordination from hexacoordination to pentacoordination, reducing bond length variation from 0.204 to 0.209 nm during NH4+ intercalation (Figure 8D), strengthening the ZnCuHCF framework and thus leading to an ultralong cycle life with a retention of 92.1% after 10,000 cycles in a 23 m NH4OTf + 0.5 m Zn(OTf)2 hybrid electrolyte (Figure 8E). Besides, the ZnCuHCF/CNTF delivered a high discharge potential of 0.94 V, a specific capacity of 121.7 mAh g−1 at 1 A g−1 (Figure 8F).
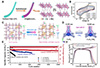 |
Figure 8 Research progress of FAABs. (A) Schematic illustration of the CF@urchin-like NH4V4O10. (B) CV curve of the FAABs. (A, B) Reproduced with permission from [94]. Copyright©2020, Elsevier. (C) Schematic illustration of in-situ dynamic compensation strategy. (D) Theoretical calculations of Cu–N bond length changes during ammoniation/de-ammoniation progresses in ZnCuHCF. (E) Cycling performance of the ZnCuHCF. (F) The GCD curves of ZnCuHCF. (C–F) Reproduced with permission from [95]. Copyright©2025, Wiley-VCH. |
These studies underscore the feasibility and potential of FAABs as emerging candidates for fiber-shaped energy storage in flexible and wearable applications. The progress made from material design to device-level demonstrations has laid a solid foundation for this burgeoning field. Nevertheless, realizing the full potential of FAABs still requires overcoming several critical challenges. Future efforts should focus on developing advanced NH4+ host materials with both high capacity and mechanical compliance, optimizing ion-conductive yet robust gel electrolytes, and establishing scalable fabrication techniques compatible with textile integration. With continued innovation across materials, structures, and device engineering, FAABs are poised to become a key enabling technology for next-generation smart wearables.
Fiber-shaped alkaline aqueous batteries
Compared with fiber-shaped aqueous ion batteries that primarily employ neutral or mildly acidic electrolytes, research on alkaline systems remains in their infancy [96,97]. Nevertheless, such systems exhibit unique advantages in terms of reaction kinetics and energy density. In alkaline media, zinc possesses a relatively high hydrogen evolution overpotential, which suppresses side reactions and thereby enhances Coulombic efficiency [98,99]. At the same time, Co/Ni-based oxides and hydroxides demonstrate superior electrochemical activity and stability in alkaline electrolytes. Consequently, despite the limited number of reports, alkaline FABs hold significant potential for high-energy-density and high-power flexible energy supply scenarios.
Current research on alkaline FABs has mainly focused on two representative systems: fiber-shaped zinc-air batteries (FZABs) and alkaline fiber-shaped rechargeable Zn-/Ni-based batteries (e.g., Zn-Co, Zn-Ni, and Ni-Fe). FZABs operate through a coupled electrochemical process, where zinc oxidation at the anode (Zn → Zn2+ + 2e−) is paired with oxygen reduction and evolution reactions (ORR/OER) at the air cathode during discharge and charge, respectively. The reversible redox processes occur at the solid-liquid-gas triple-phase interface, which enables high theoretical energy density yet also imposes challenges of sluggish reaction kinetics and interfacial instability. Typically, metallic zinc wires or zinc-coated fibers serve as the anode, while carbon filaments or woven carbon ribbons are employed as air electrode substrates. These substrates are further integrated with bifunctional catalysts (e.g., MnOx, Co/N-doped carbon, Co-Fe oxides, and CNT/graphene composites) [100–102] to accelerate ORR and OER processes. Distinct from their planar counterparts, fiber architectures require the air electrode to be lightweight, flexible, and air-permeable, ensuring efficient oxygen diffusion and stable operation under mechanical deformation. Structural innovations, including coaxial architectures, core-sheath configurations, and hydrophobic modifications, help maintain efficient gas diffusion and prevent electrolyte flooding, enabling stable operation under mechanical deformation. These unique structural characteristics endow FZABs with exceptional adaptability for integration into wearable and textile-based energy systems, positioning them as a pivotal subclass of fiber-shaped aqueous batteries. Xu et al. [103] constructed a novel air electrode consisting of directionally stacked porous CNT sheets, which simultaneously function as a gas diffusion layer, catalyst layer, and current collector (Figure 9A, B). This architecture delivers excellent charge-discharge performance even at high current densities of 2 A g−1 (Figure 9C) and maintains stable output under bending and stretching, highlighting its promise for portable and wearable electronics. However, conventional hydrogel electrolytes rapidly lose elasticity in strong alkaline environments, thereby limiting the development of highly stretchable FZABs. To address this challenge, Ma et al. [104] developed an alkaline-tolerant dual-network hydrogel electrolyte, enabling the first realization of ultra stretchable FZABs (Figure 9D). The hydrogel, constructed from sodium polyacrylate chains and a cellulose backbone, retained excellent mechanical properties under harsh alkaline conditions. The resulting device not only exhibited a high-power density of 108.6 mW cm−2 under static conditions but also reached 210.5 mW cm−2 under extreme stretching (Figure 9E), while maintaining stable electrochemical performance after severe deformation. This breakthrough lays the groundwork for FZABs in smart textiles and flexible electronics.
 |
Figure 9 Research progress of alkaline aqueous batteries. (A) Schematic illustration of the fabrication of the FZABs. (B) Schematic illustration to the structure of the aligned CNT in the FZABs. (C) GCD curves of a FZABs at current densities of 1 A g−1. (A–C) Reproduced with permission from [103]. Copyright©2015, John Wiley and Sons. (D) Schematic illustration of 500% stretchable. (E) Power density curves of fiber-shaped highly stretchable FZABs at fully released state and 500% tensile strain with PANa-cellulose hydrogel electrolyte. (D, E) Reproduced with permission from [104]. Copyright©2019, John Wiley and Sons. (F) Schematics of the all-solid-state fiber-shaped Zn-Co battery. (G) Ragone plot of the all-solid-state fiber-shaped Zn-Co battery based on the volume of the whole flexible battery. (F, G) Reproduced with permission from [105]. Copyright©2019, John Wiley and Sons. (H) Schematic diagram of the flexible quasi-solid-state fiber-shaped Ni-NiO//Zn battery. (I) Cycling performance of the quasi-solid-state fiber-shaped Ni-NiO//Zn battery at various current densities. (H, I) Reproduced with permission from [106]. Copyright©2017, John Wiley and Sons. (J) Schematics of the fiber-shaped NiCo-Fe battery. (K) GCD curves of the fiber-shaped NiCo-Fe battery. (J, K) Reproduced with permission from [22]. Copyright©2019, John Wiley and Sons. |
Beyond FZABs systems, alkaline Zn-Co batteries have also demonstrated the advantages of fiberization. Li et al. [105] addressed zinc dendrite-induced cycling degradation by designing ZnO@C core-shell nanorods as dendrite-resistant anodes, coupled with dendritic Co(CO3)0.5(OH)x·0.11H2O@CoMoO4 cathodes. Integrated with a gel electrolyte, the team successfully fabricated a highly customizable all-solid-state fiber-shaped Zn-Co battery (Figure 9F). The device delivered an energy density of 4.6 mWh cm−3 and a peak power density of 0.42 W cm−3, retained 82% capacity after 1600 cycles, and preserved excellent flexibility under diverse bending conditions (Figure 9G). Despite their promise, the practical deployment of Zn-Ni batteries remains hindered by limited cycling stability, arising mainly from the irreversibility of nickel-based cathodes and the dendritic growth of zinc anodes. To address this challenge, Zeng et al. [106] engineered Ni-NiO heterostructure nanosheets as the cathode to construct a highly rechargeable, flexible fibrous NiZn battery with outstanding electrochemical properties (Figure 9H). The synergistic enhancement of conductivity and electroactivity imparted by the Ni-NiO heterostructure enabled the device to deliver high capacity and remarkable rate capability. In aqueous electrolytes, the battery retained 96.6% of its capacity after 10,000 cycles, while in polymer electrolytes it showed negligible capacity decay even after 10,000 cycles at a current density of 22.2 A g−1, setting a benchmark for cycling durability (Figure 9I). In addition, aqueous rechargeable Ni-Fe batteries, with their ultra-flat discharge plateau, low cost, and intrinsic safety, hold strong promise for wearable energy storage. Yang et al. [22] engineered S-doped Fe2O3 nanowire arrays (NWAs) grown directly on CNTF (S-Fe2O3 NWAs/CNTFs), where S doping narrowed the band gap of Fe2O3 and markedly improved its conductivity. Coupled with a Zn-Ni-Co oxide (ZNCO)@Ni(OH)2 NWAs heterostructures cathode (Figure 9J), the resulting quasi-solid-state fiber-shaped NiCo-Fe battery achieved a capacity of 0.46 mAh cm−2 (Figure 9K), surpassing most state-of-the-art fiber-shaped aqueous batteries and charting a new path for next-generation wearable Ni-Fe energy storage.
Overall, fiber-based architecture greatly enhances specific surface area and shortens ion diffusion pathways, while porous carbon frameworks facilitate gas transport and by-product removal. As a result, devices maintain high power density and reversibility even under bending, knotting, and weaving conditions. Leveraging the weavability of fiber electrodes, such batteries can be seamlessly integrated into textiles, making them suitable for powering pulsed or intermittent wearable loads. Nonetheless, alkaline systems face several critical challenges: (i) the corrosive nature and water activity of strong alkaline electrolytes compromise the chemical stability of packaging materials, current collectors and binders, thereby shortening device lifetime and raising safety concerns; (ii) zinc anodes remain susceptible to self-corrosion and morphological evolution in alkaline conditions, necessitating strategies such as alloying, protective interfacial layers and 3D porous scaffolds to suppress dendrite growth; (iii) the structural stability of bifunctional catalysts and the oxidation resistance of carbon supports require further improvement, while catalyst pore distribution and hydrophobicity strongly influence water management and reaction pathways; (iv) strain concentration during bending, stretching and weaving can induce active-layer delamination and interfacial impedance growth, leading to performance fluctuations and efficiency loss. Therefore, future research urgently needs to achieve synergistic breakthroughs in material design, electrolyte regulation and device structure optimization. With the continued progress of highly stable catalysts, solid-state alkaline electrolytes and alkali-resistant packaging technologies, alkaline fiber batteries are expected to achieve a leap forward from laboratory verification to prototypes for smart textile and flexible electronics applications.
FABs have emerged as a highly promising class of energy storage devices, offering distinctive advantages such as intrinsic safety, mechanical flexibility, and compatibility with wearable and textile electronics. In recent years, research efforts have focused on a variety of chemistries, including monovalent-ion systems (Li+, Na+), multivalent-ion systems (Zn2+, Mg2+, Ca2+, Al3+), and alkaline FABs. Monovalent systems, particularly those based on lithium and sodium, exhibit relatively mature electrochemical performance with well-understood reaction mechanisms and stable cycling behavior. However, their practical deployment is limited by safety concerns associated with dendrite growth, the high cost of Li-based materials, and the restricted energy density of Na systems. In contrast, multivalent-ion systems provide higher theoretical capacity and volumetric energy density due to the multiple-electron transfer per cation, along with superior resource abundance and environmental benignity. Nevertheless, the strong coulombic interactions and rigid hydration shells of multivalent cations lead to sluggish ion diffusion, interfacial polarization, and structural degradation, which collectively restrict their rate capability and reversibility. FAABs have recently gained attention owing to their unique hydrogen-bond-assisted transport mechanism, enabling fast ion mobility and high reversibility in mild aqueous environments. Yet, their relatively low redox potential and limited energy output remain obstacles to large-scale application. Although early in development, alkaline FABs exhibit compelling advantages in kinetics and energy density, positioning them as a promising complementary strategy. Despite these advances, the practical development of FABs remains hindered by several common bottlenecks. Future progress in fibrous aqueous ion batteries will therefore depend on the integration of materials innovation, electrolyte engineering, and structural design. Moreover, with continuous advances in materials science, electrochemistry, and device integration, FABs are expected to play a pivotal role in powering next-generation wearable electronics, smart textiles, and implantable medical devices.
Comparative analysis of FABs systems
The exploration of various FABs systems reveals a diverse landscape where each chemistry is propelled by distinct advantages and, consequently, faces unique developmental bottlenecks. This comparative analysis synthesizes the core value proposition and the primary limiting factor for each system, providing a high-level perspective on their respective developmental trajectories (Table 2).
Comparative summary of development status and key challenges for different fiber-shaped aqueous battery systems
Monovalent-ion systems (Li+, Na+) are primarily developed as a safe and flexible translation of mature battery chemistry. Their development is constrained not by fundamental electrochemical unknowns, but by the practical limitations of the aqueous electrolyte’s narrow voltage window and, for lithium, resource considerations.
Zinc-ion systems (Zn2+) are at the forefront of multivalent FABs development, driven by the pursuit of high volumetric energy density and exceptional practicality. The central bottleneck for this system is unequivocally the interfacial instability at the zinc anode (dendrites, side reactions) alongside the search for cathode materials that can withstand sustained Zn2+ (de)intercalation.
Other multivalent-ion systems (Mg2+, Ca2+, Al3+) represent the quest for ultra-high theoretical capacity and resource sustainability. Their development is in its infancy, fundamentally hindered by the sluggish solid-state diffusion kinetics of the highly polarizing multivalent ions within most host materials.
Ammonium-ion systems (NH4+) offer a pathway to ultra-fast charging and unique sustainability. As an emerging field, its progress is currently gated by the limited library of high-performance host materials, especially for the anode, which can leverage the unique chemistry of the NH4+ ion.
Alkaline systems (e.g., Zn-air, Ni-Zn) are engineered for high power and energy density in a unique chemical environment. Their development is uniquely challenged by the need for component stability and durability within the corrosive alkaline electrolyte.
This differentiation in core challenges dictates divergent research priorities for each system, as will be further discussed in the context of future prospects.
APPLICATION
FABs, leveraging their excellent mechanical properties, sewability, diversified charging modes, and multifunctional integration capabilities, precisely meet the core demands of wearable electronics and smart textiles for morphological compatibility, self-powered capability and functional synergy, thus emerging as a research hotspot in this field [1,28,97]. From the optimization of basic structure-performance relationships to the integration of complex multi-systems, FABs research have consistently focused on overcoming bottlenecks in practical application scenarios, following a clear progressive development pathway of basic performance - energy supply - functional synergy - miniaturized integration: First, it addresses the morphological compatibility issue through material and structural innovations to achieve seamless integration with textiles; second, it expands charging modes to break the limitations of energy supplementation and enhance environmental adaptability; third, it realizes energy storage-functional synergy via heterogeneous device coupling to enrich the application dimensions of smart textiles; finally, it moves toward multifunctional miniaturized integration in a single fiber to promote the evolution of wearable devices toward lightweight and unobtrusive designs.
Mechanical properties and sewability
Wearable electronics require long-term conformity to dynamic human movements and compatibility with textile processing (sewing) and usage (washing) scenarios [1,27,69,107]. This demands that energy storage devices not only possess excellent flexibility, stretchability, and bending resistance but also high adaptability to textile manufacturing processes. Through a dual-driver approach of material design and structural innovation, FABs precisely overcome bottlenecks in mechanical performance, laying a morphological foundation for subsequent multifunctional integration, which serves as the core prerequisite for FABs to transition from laboratory prototypes to practical textile components.
In terms of material selection, the combination of flexible conductive materials and high-toughness electrolytes is key to enhancing mechanical performance: The introduction of materials such as carbon nanotubes (with high conductivity and flexibility), metal-organic frameworks (MOFs, featuring high specific surface area and tunable structure) [27] and conductive polymers significantly improves the tensile and bending resistance of electrodes [108]; polymer gel electrolytes prevent the leakage of liquid electrolytes through the entanglement of polymer networks, while simultaneously enhancing the overall flexibility and swelling resistance of FABs.
In structural design, innovative configurations, including coaxial, twisted, and coated structures, further optimize the stress distribution of fibers. For instance, the FABs developed by Kareri et al. [109]—using a polyaniline-based photoactive gel as the electrolyte and conductive threads as the electrodes—forms a fiber structure by twisting the electrode threads at a density of 10 twists per cm (Figure 10A). This twisted design not only disperses local stress during deformation but also allows the device to be directly embedded into textile textures, perfectly meeting the demand for unobtrusive integration in wearable devices. In addition, Li et al. [81] developed FABs based on double-helical structured electrodes and cross-linked polyacrylamide electrolytes, which exhibit outstanding mechanical elasticity and deformation tolerance: the stretchability reaches 300%, the capacity retention rate exceeds 95% after non-planar deformations, and the capacity retention remains at 98.5% after 500 cycles (Figure 10B). These properties enable stable adaptation to the dynamic deformation requirements of wearable devices.
 |
Figure 10 Research progress of FABs. (A) Schematic illustration of the fabrication steps of the Ny66-Ag/CNT/ZnO-NWs anode electrode and the final device structure. Reproduced with permission from [109]. Copyright©2022, The Author(s). (B) Schematic diagram of fabrication and encapsulation of FABs. Reproduced with permission from [81]. Copyright©2018, American Chemical Society. (C) The performances of sewn and woven power textiles based on the FABs. Reproduced with permission from [110]. Copyright©2020, John Wiley and Sons. (D) Schematic of the FABs with air-recharging capability integrated into multifunctional wearable systems. Reproduced with permission from [114]. Copyright©2021, Royal Society of chemistry. (E) Cycling performance with or without Mn2+ in the electrolyte of FABs. (F) The photo-rechargeable fabric delivered stable electrical output under varying environmental disturbance. (E, F) Reproduced with permission from [116]. Copyright©2020, Elsevier. (G) The performance of wearable sensor device integrated with FABs. Reproduced with permission from [121]. Copyright©2022, Elsevier. (H) I-T curves of the device at different incidence angles. Reproduced with permission from [85]. Copyright©2023, American Chemical Society. (I) Fluorescent display for FABs. Reproduced with permission from [84]. Copyright©2023, American Chemical Society. |
Notably, washability is one of the key indicators for FABs to achieve practical applications. Traditional energy storage devices, due to their water-sensitive packaging, cannot withstand the machine washing process of textiles. In contrast, the sewable FABs developed by Wang et al. [110], modified with a swelling-resistant polymer gel electrolyte and a double-layer sealed packaging design, can be directly woven into textiles as a thread (Figure 10C). After thousands of bending cycles and repeated machine washes, it maintains a capacity retention rate of over 90% without significant degradation in power supply stability. This breakthrough addresses the service durability challenge in the large-scale industrialization of FABs, while also verifying the practicality of Zn-MOF-based FABs in powering small wearable devices such as calculators and electronic watches [1], thus providing a reliable morphological carrier for subsequent energy supply and functional integration.
Integration of diversified charging modes
While optimized mechanical properties solve the morphological compatibility issue of FABs with textiles, energy supplementation for wearable devices in scenarios without external power sources (outdoor activities, sports, and emergencies) remains a core pain point [111]. Traditional wired charging is not only inconvenient but also limits the mobility of devices. By integrating novel charging modes such as air-charging and photo-charging, FABs achieve the goal of energy harvesting from the environment, significantly expanding their application boundaries and providing an energy guarantee for the long-term stable operation of multifunctional systems [111–113].
Air-charging FABs are characterized by their structural simplification through the use of environmental resources: By introducing an air cathode, they utilize O2 in the air as a redox-active species, eliminating the need for additional energy storage electrodes. This design not only improves energy density but also reduces device weight. For example, Liao et al. [114] reported a FAB with a V6O13/oriented carbon nanotube composite cathode (VCF) as the core component. Air charging is realized through the spontaneous oxidation reaction between the discharged VCF and O2 in the air (accompanied by Zn2+ deintercalation, Figure 10D). To address electrolyte volatilization and impurity erosion in open environments, the team adopted a double-layer tubular packaging design (inner layer for electrolyte retention, outer layer for contamination prevention), ensuring stable air charging in complex environments such as outdoor settings and humid conditions. This design effectively mitigates the power failure risk of wearable electronics in scenarios like outdoor exploration and emergency rescue, enhancing the environmental robustness of devices.
Photo-charging FABs, on the other hand, focus on high-efficiency energy conversion. By integrating a photoelectric conversion unit into the fiber structure, they directly convert ambient light into electrical energy for storage, enabling the integrated conversion and storage of light energy as electrical energy. Xiong et al. [115] developed an all-in-one photo-powered FABs using a MoS2@TiO2@Ti composite cathode (MoS2 enhances photoelectric response, while TiO2 improves stability). This battery can be charged by natural light without an external power source, simplifying the separate circuit of a photovoltaic cell to an energy storage battery.
Furthermore, Zhang et al. [116] achieved deep integration of photovoltaic components and energy storage batteries to develop a photo-charging textile. This textile uses a ZnO nanowire array-based baseline photoanode as the energy-harvesting unit and a Zn/MnO2 FABs as the energy-storage unit (Figure 10E). It can be charged under sunlight for only one minute and then stably discharge at a current of 0.1 mA for 10 min, with photoelectric conversion and energy storage efficiency significantly superior to that of traditional separate systems (Figure 10F).
It is worth emphasizing that air charging and photocharging modes exhibit significant complementarity: The former is suitable for scenarios with insufficient light but good air circulation (indoors, cloudy days and nights), while the latter is applicable for light-abundant scenarios (outdoors and daytime). The synergistic integration of the two (via a dual-cathode design) is expected to achieve all-weather self-powering, further reducing the dependence of wearable devices on external power sources. With the gradual resolution of morphological compatibility and energy supply issues, FABs have begun to advance toward the higher-level energy storage-functional synergy—that is, endowing smart textiles with more practical functions through heterogeneous coupling with sensing and detection devices.
Multifunctional integration and heterogeneous coupling
The core value of wearable electronics lies not only in energy storage but also in realizing specific functions based on energy. By means of heterogeneous integration with strain, pressure, and photoelectric sensing devices, FABs have constructed energy storage-signal sensing integrated systems, promoting the transformation of smart textiles from single energy storage carriers to multifunctional smart terminals [117]. They exhibit irreplaceable application potential, especially in fields such as health monitoring, human-computer interaction, and environmental sensing.
The key to such integrated systems lies in the synergy between material compatibility and performance matching: On one hand, batteries and sensors need to adopt similar flexible substrates to ensure that the integrated system still maintains excellent mechanical properties; on the other hand, the output voltage and current of batteries must match the operational requirements of sensors to avoid signal distortion caused by unstable power supply [118,119].
In terms of motion signal monitoring, the FABs developed by Zhang et al. [117] (with an energy density of 396 Wh kg−1 and a capacity retention rate of 80.6% after 300 cycles), when integrated with a carbon nanotube/PDMS strain sensor, can be attached to parts such as the wrist, fingers and knee joints to real-time capture the bending angle and movement frequency of human joints. Its high energy density ensures long-term power supply for the sensor, while its excellent flexibility avoids discomfort during movement. Liu et al. [93], by contrast, selected a fiber-shaped aqueous Ca2+ battery (with an energy density of 43.2 mWh cm−3) for combination with a strain sensor. They utilized the low polarization property of Ca2+ to enhance the battery’s stability under repeated deformation, making it suitable for signal acquisition in long-duration sports scenarios such as marathons and cycling.
For subtle physiological signals such as pulse, voice, and swallowing, researchers mostly choose supercapacitors with high power density as the energy unit. For example, Ma et al. [120] used an aqueous MXene fiber supercapacitor (featuring fast charge-discharge performance and high power density) to power a pressure sensor, constructing a self-powered monitoring system. The high conductivity of MXene ensures the instantaneous response of the pressure sensor (capable of detecting pressure changes below 1 Pa), enabling the system to accurately identify pulse waveforms, laryngeal vibration during speech and swallowing movements—providing a new tool for cardiovascular health assessment and assisted diagnosis of speech disorders.
In addition, Li et al. [121] developed a FAB with an amorphous H0.82MoO3.26-coated carbon nanotube fiber cathode, which combines high energy density, cycling stability, and flexibility (Figure 10G). Its integrated system with a multimodal sensor can simultaneously respond to laryngeal vibration (vibration signals) during speech and finger movement (strain signals), realizing dual-mode human-computer interaction of voice control + gesture interaction and further expanding the application dimensions of smart textiles.
The breakthroughs in these heterogeneous integrated systems not only verify the feasibility of FABs as core energy supply units for wearables but also promote the upgrade of smart textiles from passive energy storage to active sensing. However, with the increase in the number of integrated devices, the problems of volume accumulation and complex wiring have gradually become prominent. How to realize more functions in a smaller space has become the next core direction of FAB research.
Multifunctionalization and miniaturized integration of single fibers
To achieve lightweight, unobtrusive, and seamless wearability of wearable devices, researchers have begun to explore the technical pathway of a single fiber carrying multiple functions. By precisely arranging functional units (such as energy storage, photoelectric detection, and luminescent display) inside or on the surface of a single fiber, a miniaturized and integrated smart fiber system is constructed—fundamentally solving the problems of volume redundancy and complex wiring in multi-device integration. This has become a cutting-edge trend in the multifunctional integration of FABs [64,112].
The core challenge of multifunctionalization in a single fiber lies in the compatible integration of functional units: It is necessary to realize the coordinated arrangement of different functional materials at the nanometer to micrometer scale, while ensuring the independent and efficient operation of each unit (avoiding electrical interference between energy storage units and sensing units). Current research mainly overcomes this challenge through two strategies: structural design and material derivation.
Lu et al. [85] integrated a high-sensitivity photoelectric detector and a high-energy aqueous battery into a single fiber via a three-strand twisted structure—one strand serves as a ZnO-based photoelectric detection unit (with a photoresponse performance of 151.45 mA W−1) and the other two strands act as FABs units (with a capacity of 18.75 mAh cm−3, Figure 10H). The twisted structure separates each unit via an insulating layer, which not only ensures electrical independence but also enhances the overall flexibility through close contact between fibers, enabling the device to simultaneously realize ambient light intensity monitoring and energy storage.
Pu et al. [122] used a vanadium-based metal-organic framework nanowire@carbon nanotube fiber (V-MOF NWs@CNT) as the core derived matrix. Through stepwise pyrolysis and electrochemical modification, they prepared a VN NWs@CNT fibrous piezoresistive sensor and a V-MOF-derived FABs, respectively. Since both are based on the same carbon nanotube fiber matrix, they can be directly integrated into a single fiber to form an energy supply-sensing integrated system.
Beyond energy supply-sensing integration, FABs can also be combined with display units to expand the interaction dimensions of smart textiles. For example, Liu et al. [84] introduced fluorescent carbon dots into an FAB to construct an energy storage-multicolor luminescence integrated device (Figure 10I). After 1500 cycles, the FAB still maintains a capacity retention rate of 78.9% and can be directly woven into clothing—it not only meets daily power supply needs but also realizes nighttime safety warning, fashionable decoration, and simple human-computer interaction (feeding back device power via luminescent color).
CHALLENGES AND PROSPECTS OF FABS
As a core energy component of flexible electronic devices, FABs show broad application prospects due to their excellent safety and environmental compatibility. However, their industrialization process still faces multi-dimensional technical bottlenecks (Figure 11). In-depth analysis of these challenges and exploration of breakthrough paths are of great significance for promoting their practical application.
 |
Figure 11 Challenges of large-scale industry production of FABs. |
Challenges
Electrode material utilization
Electrode material utilization is a core parameter determining the energy density and cycle life of FABs and its performance directly dictates the practical application value of the devices. Traditional electrode materials generally exhibit low utilization rates in fiber structures, with the key limiting factors including uneven distribution of active materials on the fiber substrate, blocked electron/ion transport pathways, and coverage of active sites as well as increased interfacial impedance caused by binders. To address this bottleneck, researchers have developed a variety of targeted strategies in recent years: constructing low-dimensional morphologies such as nanowires, nanorods, and nanosheets significantly increases the exposed area of active materials, thereby enhancing their contact efficiency with electrolytes and reaction activity. Furthermore, binder-free in-situ growth technology effectively reduces the proportion of inactive components by growing active materials in-situ on the fiber substrate surface, while the construction of heterogeneous structures (e.g., core-shell structures, layered structures) further optimizes electron/ion transport kinetics, providing an effective approach to improve the overall utilization rate of electrodes. However, the further improvement of material utilization is still constrained by the size effect of fibrous structures—the small diameter of fibers tends to limit the loading capacity of active materials. Meanwhile, issues such as poor interfacial contact and detachment of active materials during cycling still require targeted solutions. To push the boundaries of material utilization, emerging strategies are focusing on advanced nanostructuring and computational design. For instance, hierarchical pore engineering utilizing techniques like electrospinning and ice-templating can create multi-scale pores to drastically increase the accessible surface area. Moreover, machine learning-driven topology optimization is being explored to design optimal electrode architectures that maximize active material loading and minimize transport barriers, potentially pushing utilization rates beyond 90%. The integration of 2D conductive additives like MXene or graphene also shows promise in enhancing electron transfer pathways and mitigating active material detachment during deformation.
Interface stability
Interface stability is a critical prerequisite for ensuring the cycle life and operational safety of FABs; its failure can directly lead to battery capacity fading, internal resistance increase, and even short-circuit risks. In aqueous systems, the interface problems of metal anodes such as Zn and Fe are particularly prominent: during cycling, uneven deposition easily occurs on the metal surface, forming dendrite structures. Dendrite growth not only consumes active materials and causes capacity loss but may also pierce the separator and trigger battery short circuits. At the same time, side reactions between the metal anode and electrolyte (e.g., hydrogen evolution reaction and surface oxidation of Zn) as well as volume expansion during charge-discharge cycles tend to cause cracks and detachment at the electrode/electrolyte interface, further exacerbating interface failure. To tackle these challenges, interface engineering strategies have become a research focus: surface coating technology can effectively inhibit dendrite growth and side reactions by constructing dense protective layers (e.g., polymer gels, inorganic coatings, functionalized carbon materials) on the surface of metal anodes. Electrolyte regulation methods (e.g., adding ion modifiers, developing gel electrolytes, constructing dual-solvent systems) improve interface stability by optimizing the interfacial ion transport environment. Additionally, interface regulation theories based on defect engineering and element doping, combined with dynamic monitoring via in-situ characterization techniques, can further reduce the ion diffusion barrier and enhance the reversibility of interfacial reactions, providing theoretical support for the optimization of interface stability. Looking forward, solutions are evolving towards more intelligent and precise interface control. The development of smart electrolyte systems with stimulus-responsive components (pH-sensitive hydrogels) that can autonomously release functional ions or form protective barriers at the onset of dendrite formation represents a promising direction. At the material level, atomic-level coating techniques such as atomic layer deposition can create ultrathin, conformal, and robust artificial solid electrolyte interphase layers for superior inhibition of side reactions. Furthermore, coupling real-time in-operando characterization techniques with machine learning models can dynamically diagnose interface failure mechanisms, paving the way for feedback-controlled and self-adaptive interfaces.
Encapsulation and integration
The encapsulation and integration of FABs need to simultaneously meet four core requirements: flexibility, airtightness, durability, and weavability, and this process faces multiple technical contradictions and application bottlenecks. From the perspective of flexibility and mechanical properties, traditional metal current collectors and rigid encapsulation materials (e.g., metal foils, rigid polymers) are difficult to adapt to the dynamic application scenarios of wearable devices. They are prone to structural damage under mechanical deformations such as bending and stretching, leading to electrolyte leakage or electrode short circuits. From the perspective of environmental adaptability, batteries need to cope with complex working conditions such as washing, temperature fluctuations, and humidity changes in practical applications. The insufficient airtightness and weather resistance of traditional encapsulation schemes easily cause device performance degradation or even failure. To solve these problems, novel encapsulation materials and structural designs have become research priorities: the application of flexible conductive materials such as carbon nanotube fibers, conductive polymers, and ultra-soft hydrogels significantly improves the mechanical tolerance of batteries while ensuring conductivity. Microstructural designs such as coaxial structures and core-shell structures effectively optimize the space utilization and structural stability of devices by integrating electrodes, electrolytes, and encapsulation layers into a single unit. Moreover, the multi-functional integration strategy (e.g., combining energy storage with sensing, luminescence, and energy harvesting functions) provides new ideas for the system-level integration of smart textiles, promoting the transformation of FABs from single energy devices to multi-functional smart components. The next wave of innovation aims for seamless textile compatibility and multi-functionality through advanced manufacturing. Emerging technologies like modular and programmable encapsulation using thermoplastic elastomers (TPEs) or shape-memory polymers enable customizable and repairable packaging tailored to specific textile processes. Moreover, the advancement of multi-material thermal drawing technique promises the co-drawing of polymers, metals, and composites into kilometer-long, hermetically sealed core-shell fibers, which is ideal for scalable production of integrated devices.
Economic viability
Economic viability is a key factor restricting the transition of FABs from laboratory research to commercialization and large-scale application, with its core contradictions focusing on two aspects: material costs and production process costs. At the material level, the preparation processes of current high-performance electrodes (e.g., high-purity nanocarbon materials, noble metal-doped active materials) and electrolytes (e.g., functionalized ionic liquids, high-purity salts) are complex, with harsh reaction conditions. In addition, some key raw materials (e.g., high-quality graphene, rare metal catalysts) are expensive, resulting in high unit device costs. At the production process level, the preparation of FABs has not yet formed a unified standardized process; the process parameters for spinning, coating, integration, and other links lack standardization. During mass production, problems such as poor product consistency and low yield easily occur, further increasing the industrialization costs. Therefore, future improvements in economic viability need to achieve breakthroughs in two aspects: firstly, developing low-cost, high-performance alternative materials (e.g., biomass-derived carbon materials, transition metal oxides) to reduce raw material costs while ensuring performance; secondly, optimizing production processes, developing continuous and automated preparation equipment and establishing standardized production processes to improve the efficiency of mass production and product qualification rates. Concrete pathways to enhance economic viability are centered on sustainable materials and scalable manufacturing. Leveraging biomass-derived carbons (from lignin, cellulose) and earth-abundant transition metal compounds can serve as low-cost, high-performance alternatives. On the production front, implementing high-throughput processes like roll-to-roll production lines and microfluidic spinning technologies is key to reducing labor and time costs through continuous, automated fabrication. Furthermore, establishing industry consortia to define material specifications and process standards can streamline the supply chain, reduce overhead, and drive down costs via economies of scale.
Standardization
Standardization is the fundamental support for promoting the industrialization of FABs and a prerequisite for realizing horizontal comparison of technical achievements and standardized market development. Currently, there is no unified system of performance evaluation standards, testing methods, and safety specifications in this field, resulting in poor comparability and reference value of experimental results among different research teams. For example, in energy density testing, some studies calculate based on “electrode mass energy density” while others use “full-cell volume energy density” and there is no unified standard for parameters such as whether electrolyte dosage and current collector mass are included. In cycle life evaluation, differences in charge-discharge rates, cut-off voltages, and cycle termination conditions can also lead to deviations in results. Furthermore, the lack of methods and criteria for flexible performance testing (e.g., bending angle, stretching rate, cyclic deformation times) and safety testing (e.g., short-circuit testing, extrusion testing, electrolyte leakage testing) further hinders the promotion of technical achievements and market application. Therefore, establishing a multi-dimensional standard system covering energy density, power density, cycle life, flexibility, and safety, and clarifying testing methods and evaluation indicators, has become an important direction for the industrial development of FABs.
Device performance
The device performance of FABs is a direct reflection of their application value, covering key indicators such as energy density, power density, cycle life, rate performance, flexibility, and safety. Currently, the performance improvement in this field presents the characteristic of “partial breakthroughs and local shortcomings”. In terms of energy density and cycle life, through strategies such as binder-free electrode design, interface engineering optimization, and integration of high-loading active materials, the performance of some FABs has approached or even exceeded that of traditional small lithium-ion batteries. However, there is still significant room for improvement in high-rate performance and adaptability to extreme environments. For instance, under high-rate charge-discharge conditions, the electron/ion transport bottleneck of fibrous electrodes easily leads to a sharp decline in capacity. In low-temperature (<0 °C), high-temperature (>60 °C) and high-humidity environments, issues such as reduced ionic conductivity of electrolytes and slow electrode reaction kinetics significantly affect the stability of device performance. In addition, in practical wearable scenarios, batteries need to withstand complex mechanical deformations such as repeated bending, stretching, and twisting for a long time. The current device performance retention rate is still low and the coordinated stability between mechanical deformation and electrochemical performance is insufficient, which has become a core shortcoming restricting its practical application.
Future prospects
Facing the next generation of flexible electronic applications, the development of FABs will focus on the following directions to achieve the leap from laboratory to industrialization.
Innovation of intelligent material systems
Topology optimization design driven by machine learning will significantly enhance electrode performance. For instance, graphene aerogel fibers optimized via neuromorphic algorithms can precisely regulate pore distribution, enabling the utilization rate of active materials in porous electrodes to exceed 90%. Bionic electrolyte engineering focuses on stimulus-responsive properties—pH-sensitive hydrogels can automatically release OH− ions to form an inhibitory layer at the initial stage of dendrite growth, which has been verified by in-situ Raman spectroscopy. Such “intelligent materials” will endow batteries with disruptive functions, such as self-diagnosis and self-repair.
Breakthroughs in manufacturing technologies
The integration of microfluidic spinning technology enables one-step continuous preparation of battery fibers with core-shell structures. The key to achieving a production speed of 200 m min−1 lies in the precise coupling of anode spinning, electrolyte coating, cathode weaving, and in-situ packaging processes. The introduction of 3D printing technology, which leverages the phase transition properties of shape memory polymers, can dynamically optimize the interfacial contact state between electrodes and electrolytes, reducing interface impedance by 65% under bending conditions.
Construction of multi-functional integration systems
The integrated design of energy-information-execution allows fibrous batteries to go beyond the function of mere energy storage units: embedded piezoelectric ZnO nanowires can real-time monitor the mechanical strain distribution during charging and discharging, thereby realizing self-perception of battery health status. This cross-domain integration promotes the evolution of batteries from “energy components” to “intelligent organs”.
Improvement of standardization and industrialization pathways
It is urgent to establish an exclusive evaluation system for flexible batteries: formulating classification standards for capacity decay rate under dynamic bending cycles (e.g., a capacity retention rate of over 80% after 10,000 cycles is designated as Grade A) and developing accelerated aging test protocols to predict performance over a ten-year service life. From the perspective of sustainable development, the full-lifecycle design of biodegradable packaging materials (such as chitosan/cellulose nanocrystal composite films) will address the issue of electronic waste. After completing their service life, batteries can achieve controlled degradation through enzymatic catalysis, ultimately forming a closed-loop chain of “green creation - intelligent application - ecological recycling”.
CONCLUSIONS
FABs have rapidly evolved into a pivotal branch of flexible energy storage technologies, offering an effective solution to the urgent demand for safety, mechanical compliance, and environmental compatibility in wearable electronics. Over the past decade, continuous advancements in electrode materials, fabrication strategies, and structural designs have enabled FABs to achieve significant improvements in energy density, power capability, cycling stability, and multifunctional integration. By leveraging aqueous electrolyte systems, FABs intrinsically avoid the flammability and toxicity issues associated with organic electrolytes, while their fiber-shaped architectures ensure superior deformability and seamless compatibility with textile platforms. Despite these promising advances, critical challenges remain. Low active material utilization, unstable electrode-electrolyte interfaces, and the lack of standardized performance evaluation systems still hinder practical deployment. Addressing these issues will require synergistic progress in several directions: (i) the development of intelligent and multifunctional electrode materials with high electrochemical reversibility and mechanical robustness; (ii) scalable, precise and environmentally friendly manufacturing techniques capable of balancing performance with cost efficiency; (iii) systematic efforts in standardization, including unified testing protocols and reliability criteria, to accelerate industrial translation.
Looking forward, FABs are expected to evolve beyond their role as single energy storage units into multifunctional platforms, enabling the integration of energy supply with sensing, display, and interactive functions. Such progress will not only drive the next generation of truly wearable, weavable, and intelligent energy fibers, but also contribute to the construction of sustainable smart societies. With continuous interdisciplinary innovation and collaborative standardization, FABs hold the potential to become a cornerstone technology for future wearable electronics and the broader landscape of flexible energy systems.
Funding
This work was supported by the National Natural Science Foundation of China (52473270, T2422028), the National Key R&D Program of China (2022YFA1203304), the Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences (start-up grant, E1552102), the China Postdoctoral Science Foundation (2024M764385, GZC20250555, GZC20250062), and the Jiangsu Funding Program for Excellent Postdoctoral Talent (2025ZB291).
Author contributions
L.H. and Y.L. wrote the manuscript. F.L. and Q.Z. revised the manuscript. All authors edited and proofread the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Zhang Q, Li C, Li Q, et al. Flexible and high-voltage coaxial-fiber aqueous rechargeable zinc-ion battery. Nano Lett 2019; 19: 4035-4042. [Article] [Google Scholar]
- He J, Lu C, Jiang H, et al. Scalable production of high-performing woven lithium-ion fibre batteries. Nature 2021; 597: 57-63. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Jia H, Wang Z, Tawiah B, et al. Recent advances in zinc anodes for high-performance aqueous Zn-ion batteries. Nano Energy 2020; 70: 104523. [Article] [Google Scholar]
- Wang Z, Huang W, Hua J, et al. An anionic‐MOF-based bifunctional separator for regulating lithium deposition and suppressing polysulfides shuttle in Li-S batteries. Small Methods 2020; 4: 2000082. [Article] [Google Scholar]
- Yun J, Kim D, Lee G, et al. All-solid-state flexible micro-supercapacitor arrays with patterned graphene/MWNT electrodes. Carbon 2014; 79: 156-164. [Article] [Google Scholar]
- Duan J, Xie W, Yang P, et al. Tough hydrogel diodes with tunable interfacial adhesion for safe and durable wearable batteries. Nano Energy 2018; 48: 569-574. [Article] [Google Scholar]
- Zhu B, Jin Y, Hu X, et al. Poly(dimethylsiloxane) thin film as a stable interfacial layer for high-performance lithium-metal battery anodes. Adv Mater 2017; 29: 1603755. [Article] [Google Scholar]
- Zeng Y, Zhang X, Meng Y, et al. Achieving ultrahigh energy density and long durability in a flexible rechargeable quasi-solid-state Zn-MnO2 battery. Adv Mater 2017; 29: 1700274. [Article] [Google Scholar]
- Li H, Ma L, Han C, et al. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019; 62: 550-587. [Article] [Google Scholar]
- Kwon YH, Woo S, Jung H, et al. Cable‐type flexible lithium ion battery based on hollow multi-helix electrodes. Adv Mater 2012; 24: 5192-5197. [Article] [Google Scholar]
- Ren J, Zhang Y, Bai W, et al. Elastic and wearable wire-shaped lithium-ion battery with high electrochemical performance. Angew Chem Int Ed 2014; 53: 7864-7869. [Article] [CrossRef] [Google Scholar]
- Fang X, Weng W, Ren J, et al. A cable-shaped lithium sulfur battery. Adv Mater 2016; 28: 491-496. [Article] [Google Scholar]
- Zhou J, Li X, Yang C, et al. A quasi-solid-state flexible fiber-shaped Li-CO2 battery with low overpotential and high energy efficiency. Adv Mater 2019; 31: 1804439. [Article] [Google Scholar]
- Song C, Li Y, Li H, et al. A novel flexible fiber-shaped dual-ion battery with high energy density based on omnidirectional porous Al wire anode. Nano Energy 2019; 60: 285-293. [Article] [Google Scholar]
- Yang J, Wang Z, Wang Z, et al. All-metal phosphide electrodes for high-performance quasi-solid-state fiber-shaped aqueous rechargeable Ni-Fe batteries. ACS Appl Mater Interfaces 2020; 12: 12801-12808. [Article] [Google Scholar]
- Liu F, Li L, Xu S, et al. Cobalt-doped MoS2·nH2O nanosheets induced heterogeneous phases as high-rate capability and long-term cyclability cathodes for wearable zinc-ion batteries. Energy Storage Mater 2023; 55: 1-11. [Article] [Google Scholar]
- Zhang Y, Wang Y, Wang L, et al. A fiber-shaped aqueous lithium ion battery with high power density. J Mater Chem A 2016; 4: 9002-9008. [Article] [Google Scholar]
- Shi J, Liu S, Zhang L, et al. Smart textile-integrated microelectronic systems for wearable applications. Adv Mater 2020; 32: 1901958. [Article] [Google Scholar]
- Liao M, Ye L, Zhang Y, et al. The recent advance in fiber-shaped energy storage devices. Adv Elect Mater 2019; 5: 1800456. [Article] [Google Scholar]
- Song WJ, Lee S, Song G, et al. Recent progress in aqueous based flexible energy storage devices. Energy Storage Mater 2020; 30: 260-286. [Article] [Google Scholar]
- Zhang Q, Zhou Z, Pan Z, et al. All-metal-organic framework-derived battery materials on carbon nanotube fibers for wearable energy-storage device. Adv Sci 2018; 5: 1801462. [Article] [Google Scholar]
- Yang J, Zhang Q, Wang Z, et al. Rational construction of self-standing sulfur-doped Fe2O3 anodes with promoted energy storage capability for wearable aqueous rechargeable NiCo‐Fe batteries. Adv Energy Mater 2020; 10: 2001064. [Article] [Google Scholar]
- Li Q, Fu J, Zhang L, et al. Long-lifespan fibrous aqueous Ni//Bi battery enabled by Bi2O3-Bi2S3 hierarchical heterostructures. ACS Appl Mater Interfaces 2024; 16: 36413-36422. [Article] [Google Scholar]
- He B, Man P, Zhang Q, et al. All binder-free electrodes for high-performance wearable aqueous rechargeable sodium-ion batteries. Nano-Micro Lett 2019; 11: 101. [Article] [Google Scholar]
- Xiong T, He B, Zhou T, et al. Stretchable fiber‐shaped aqueous aluminum ion batteries. EcoMat 2022; 4: e12218. [Article] [Google Scholar]
- Wang H, Lu Y, Nie Z, et al. Constructing carbon nanotube hybrid fiber electrodes with unique hierarchical microcrack structure for high-voltage, ultrahigh-rate, and ultralong-life flexible aqueous zinc batteries. Small 2023; 19: 2206338. [Article] [Google Scholar]
- Li C, Wang W, Luo J, et al. High‐fluidity/high‐strength dual‐layer gel electrolytes enable ultra‐flexible and dendrite‐free fiber‐shaped aqueous zinc metal battery. Adv Mater 2024; 36: 2313772. [Article] [Google Scholar]
- Jia H, Liu K, Lam Y, et al. Fiber-based materials for aqueous zinc ion batteries. Adv Fiber Mater 2023; 5: 36-58. [Article] [Google Scholar]
- Zhang N, Wang JC, Guo YF, et al. Insights on rational design and energy storage mechanism of Mn-based cathode materials towards high performance aqueous zinc-ion batteries. Coord Chem Rev 2023; 479: 215009. [Article] [Google Scholar]
- Huang Y, Mou J, Liu W, et al. Novel insights into energy storage mechanism of aqueous rechargeable Zn/MnO2 batteries with participation of Mn2+. Nano-Micro Lett 2019; 11: 49. [Article] [Google Scholar]
- Chen X, Li W, Reed D, et al. On energy storage chemistry of aqueous Zn-ion batteries: From cathode to anode. Electrochem Energy Rev 2023; 6: 33. [Article] [Google Scholar]
- Wu Y, Shuang W, Wang Y, et al. Recent progress in sodium-ion batteries: Advanced materials, reaction mechanisms and energy applications. Electrochem Energy Rev 2024; 7: 17. [Article] [Google Scholar]
- Wu Z, Ye F, Liu Q, et al. Simultaneous incorporation of V and Mn element into polyanionic NASICON for high energy‐density and long-lifespan Zn-ion storage. Adv Energy Mater 2022; 12: 2200654. [Article] [Google Scholar]
- Ye F, Pang R, Lu C, et al. Reversible ammonium ion intercalation/de-intercalation with crystal water promotion effect in layered VOPO4·2 H2O. Angew Chem Int Ed 2023; 62: e202303480. [Article] [Google Scholar]
- Chao D, Zhou W, Xie F, et al. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci Adv 2020; 6: eaba4098. [Article] [Google Scholar]
- Chen Z, Zhi C. Chalcogens for high-energy batteries. Nat Rev Mater 2025; 10: 268-284. [Article] [Google Scholar]
- Liang Y, Yao Y. Designing modern aqueous batteries. Nat Rev Mater 2023; 8: 109-122. [Article] [Google Scholar]
- Guan K, Chen W, Yang Y, et al. A dual salt/dual solvent electrolyte enables ultrahigh utilization of zinc metal anode for aqueous batteries. Adv Mater 2024; 36: 2405889. [Article] [Google Scholar]
- Liu Q, Zhang D, Yang Y, et al. Encapsulation of prussian blue analogues with conductive polymers for high-performance ammonium-ion storage. Adv Energy Mater 2025; 15: 2402863. [Article] [Google Scholar]
- Xu J, Liu Y, Xu C, et al. Aqueous non-metallic ion batteries: Materials, mechanisms and design strategies. Coord Chem Rev 2023; 474: 214867. [Article] [Google Scholar]
- Zheng W, Hu X, Wu M, et al. Recent advances and perspectives of electrode materials for emerging ammonium-ion storage: From mechanistic insights to practical applications. Chem Eng J 2023; 466: 143197. [Article] [Google Scholar]
- Zheng R, Li Y, Yu H, et al. Ammonium ion batteries: Material, electrochemistry and strategy. Angew Chem Int Ed 2023; 62: e202301629. [Article] [Google Scholar]
- Ma J, Xu M, Liu F, et al. Spatially-confined magnetite nanoparticles for superb potassium-ion storage performance. J Power Sour 2024; 596: 234102. [Article] [Google Scholar]
- Su Q, Zhang J, Wu Y, et al. Revealing the electrochemical conversion mechanism of porous Co3O4 nanoplates in lithium ion battery by in situ transmission electron microscopy. Nano Energy 2014; 9: 264-272. [Article] [Google Scholar]
- Xue X, Li Y. Advancing Mn2+/MnO2 conversion chemistry through redox mediation: Mechanistic insights and outlook. ACS Energy Lett 2025; 10: 3275-3286. [Article] [Google Scholar]
- Li Y, Li Y, Liu Q, et al. Revealing the dominance of the dissolution-deposition mechanism in aqueous Zn-MnO2 batteries. Angew Chem 2024; 136: e202318444. [Article] [Google Scholar]
- Hu J, Wang H, Wang S, et al. Electrochemical deposition mechanism of sodium and potassium. Energy Storage Mater 2021; 36: 91-98. [Article] [Google Scholar]
- Zhang Q, Zhang C, Yang F, et al. High performance fiber-shaped flexible asymmetric supercapacitor based on MnO2 nanostructure composited with CuO nanowires and carbon nanotubes. Ceram Int 2022; 48: 13996-14003. [Article] [Google Scholar]
- Huang X, Yang R, Yin H, et al. CuO@NiCo-LDH core-shell structure for flexible fiber-shaped supercapacitor electrode material. J Energy Storage 2023; 74: 109319. [Article] [Google Scholar]
- Wei S, Wan C, Huang Q, et al. 3D cellulose network confining MXene/MnO2 enables flexible wet spinning microfibers for high-performance fiber-shaped Zn-ion capacitors. Int J Biol Macromol 2024; 276: 134152. [Article] [Google Scholar]
- Lai H, Ma X, Shang P, et al. Building polyaniline:poly(styrenesulfonate)-carbon multifunctional skeleton on aerogel like substrate for high performance gel-electrolyte-friendly fiber-shaped hollow porous electrode. Mater Today Chem 2024; 41: 102293. [Article] [Google Scholar]
- Lan X, Tang T, Xie H, et al. Robust, conductive, and high loading fiber-shaped electrodes fabricated by 3D active coating for flexible energy storage devices. Nano Lett 2022; 22: 5795-5802. [Article] [Google Scholar]
- Kim J, Yu H, Jung JY, et al. 3D architecturing strategy on the utmost carbon nanotube fiber for ultra-high performance fiber-shaped supercapacitor. Adv Funct Mater 2022; 32: 2113057. [Article] [Google Scholar]
- Tian H, Liu C, Hao H, et al. Recent advances in wearable flexible electronic skin: Types, power supply methods, and development prospects. J Biomater Sci Polym Ed 2024; 35: 1455-1492. [Article] [Google Scholar]
- Wang Z, Wang Z, Li D, et al. High-quality semiconductor fibres via mechanical design. Nature 2024; 626: 72-78. [Article] [Google Scholar]
- Lu G, Xi Q, Shao Y, et al. Recent progress in carbon nanomaterials for highly flexible fibrous aqueous zinc-ion batteries. Nanoscale Adv 2024; 6: 6109-6122. [Article] [Google Scholar]
- Chen C, Feng J, Li J, et al. Functional fiber materials to smart fiber devices. Chem Rev 2022; 123: 613-662. [Article] [Google Scholar]
- Zhou X, Wang Z, Xiong T, et al. Fiber crossbars: An emerging architecture of smart electronic textiles. Adv Mater 2023; 35: 2300576. [Article] [Google Scholar]
- Fang B, Bodepudi SC, Tian F, et al. Bidirectional mid-infrared communications between two identical macroscopic graphene fibres. Nat Commun 2020; 11: 6368. [Article] [Google Scholar]
- Zhang Y, Zhao Y, Ren J, et al. Advances in wearable fiber-shaped lithium-ion batteries. Adv Mater 2016; 28: 4524-4531. [Article] [Google Scholar]
- Zhou Y, Wang C, Lu W, et al. Recent advances in fiber-shaped supercapacitors and lithium-ion batteries. Adv Mater 2020; 32: 1902779. [Article] [Google Scholar]
- Liao M, Wang C, Hong Y, et al. Industrial scale production of fibre batteries by a solution-extrusion method. Nat Nanotechnol 2022; 17: 372-377. [Article] [Google Scholar]
- Yang C, Ji X, Fan X, et al. Flexible aqueous Li-ion battery with high energy and power densities. Adv Mater 2017; 29: 1701972. [Article] [Google Scholar]
- Dai B, Shi X, Pei X, et al. Synergistic dual co-solvents hybrid electrolyte design enabling high-voltage flexible aqueous lithium-ion fiber batteries. Energy Storage Mater 2024; 66: 103231. [Article] [Google Scholar]
- Guo Z, Zhao Y, Ding Y, et al. Multi-functional flexible aqueous sodium-ion batteries with high safety. Chem 2017; 3: 348-362. [Article] [Google Scholar]
- Chen X, Wang L, Li H, et al. Porous V2O5 nanofibers as cathode materials for rechargeable aqueous zinc-ion batteries. J Energy Chem 2019; 38: 20-25. [Article] [Google Scholar]
- Volkov AI, Sharlaev AS, Berezina Ya. O, et al. Electrospun V2O5 nanofibers as high-capacity cathode materials for zinc-ion batteries. Mater Lett 2022; 308: 131212. [Article] [Google Scholar]
- Tian Y, An Y, Wei C, et al. Recent advances and perspectives of Zn‐metal free “rocking-chair”-type Zn-ion batteries. Adv Energy Mater 2021; 11: 2002529. [Article] [Google Scholar]
- Lu Y, Zhang H, Liu H, et al. Electrolyte dynamics engineering for flexible fiber-shaped aqueous zinc-ion battery with ultralong stability. Nano Lett 2021; 21: 9651-9660. [Article] [Google Scholar]
- Xiong T, Yan X, Zhang W, et al. Recent advances of aqueous fiber-shaped Zn ion batteries. Adv Fiber Mater 2025; 7: 1383-1402. [Article] [Google Scholar]
- Liu Y, Wang J, Zeng Y, et al. Interfacial engineering coupled valence tuning of MoO3 cathode for high-capacity and high-rate fiber-shaped zinc-ion batteries. Small 2020; 16: 1907458. [Article] [Google Scholar]
- Liu C, Li Q, Sun H, et al. MOF-derived vertically stacked Mn2O3@C flakes for fiber-shaped zinc-ion batteries. J Mater Chem A 2020; 8: 24031-24039. [Article] [Google Scholar]
- Zhu J, Tie Z, Bi S, et al. Towards more sustainable aqueous zinc-ion batteries. Angew Chem Int Ed 2024; 63: e202403712. [Article] [Google Scholar]
- Xiong T, Zhang Y, Lee WSV, et al. Defect engineering in manganese-based oxides for aqueous rechargeable zinc-ion batteries: A review. Adv Energy Mater 2020; 10: 2001769. [Article] [Google Scholar]
- Wang Z, Song Y, Wang J, et al. Vanadium oxides with amorphous-crystalline heterointerface network for aqueous zinc-ion batteries. Angew Chem Int Ed 2023; 62: e202216290. [Article] [Google Scholar]
- Zeng X, Gong Z, Wang C, et al. Vanadium‐based cathodes modification via defect engineering: Strategies to support the leap from lab to commercialization of aqueous zinc-ion batteries. Adv Energy Mater 2024; 14: 2401704. [Article] [Google Scholar]
- Shi Y, Yang B, Song G, et al. Ambient synthesis of vanadium-based prussian blue analogues nanocubes for high-performance and durable aqueous zinc-ion batteries with eutectic electrolytes. Angew Chem Int Ed 2024; 63: e202411579. [Article] [Google Scholar]
- Yang B, Shi Y, Song G, et al. Bimetallic design based on the nucleation rate of prussian blue analogues for enhanced aqueous zinc ion batteries with glycerol-water hybrid electrolytes. Angew Chem Int Ed 2025; 64: e202425391. [Article] [Google Scholar]
- Zeng Y, Lu XF, Zhang SL, et al. Construction of Co-Mn prussian blue analog hollow spheres for efficient aqueous Zn‐ion batteries. Angew Chem Int Ed 2021; 60: 22189-22194. [Article] [Google Scholar]
- Yu X, Fu Y, Cai X, et al. Flexible fiber-type zinc–carbon battery based on carbon fiber electrodes. Nano Energy 2013; 2: 1242-1248. [Article] [Google Scholar]
- Li H, Liu Z, Liang G, et al. Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide electrolyte. ACS Nano 2018; 12: 3140-3148. [Article] [Google Scholar]
- Li T, Xu Q, Waqar M, et al. Millisecond-induced defect chemistry realizes high-rate fiber-shaped zinc-ion battery as a magnetically soft robot. Energy Storage Mater 2023; 55: 64-72. [Article] [Google Scholar]
- Guo J, He B, Gong W, et al. Emerging amorphous to crystalline conversion chemistry in Ca‐doped VO2 cathodes for high-capacity and long-term wearable aqueous zinc-ion batteries. Adv Mater 2024; 36: 2303906. [Article] [Google Scholar]
- Liu F, Xu S, Gong W, et al. Fluorescent fiber-shaped aqueous zinc-ion batteries for bifunctional multicolor-emission/energy-storage textiles. ACS Nano 2023; 17: 18494-18506. [Article] [Google Scholar]
- Lu Z, Chen L, Zhou J, et al. Integrating high-sensitivity photodetector and high-energy aqueous battery in all-in-one triple-twisted fiber. ACS Nano 2023; 17: 20087-20097. [Article] [Google Scholar]
- Fu Q, Wu X, Luo X, et al. High‐voltage aqueous mg-ion batteries enabled by solvation structure reorganization. Adv Funct Mater 2022; 32: 2110674. [Article] [Google Scholar]
- Gheytani S, Liang Y, Wu F, et al. An aqueous Ca‐ion battery. Adv Sci 2017; 4: 1700465. [Article] [Google Scholar]
- Tao R, Fu H, Gao C, et al. Tailoring interface to boost the high-performance aqueous Al ion batteries. Adv Funct Mater 2023; 33: 2303072. [Article] [Google Scholar]
- Chen L, Bao JL, Dong X, et al. Aqueous Mg-ion battery based on polyimide anode and prussian blue cathode. ACS Energy Lett 2017; 2: 1115-1121. [Article] [Google Scholar]
- Wu C, Gu S, Zhang Q, et al. Electrochemically activated spinel manganese oxide for rechargeable aqueous aluminum battery. Nat Commun 2019; 10: 73. [Article] [Google Scholar]
- He B, Ling Y, Wang Z, et al. Modulating selective interaction of NiOOH with Mg ions for high-performance aqueous batteries. eScience 2024; 4: 100293. [Article] [Google Scholar]
- Ling Y, He B, Han L, et al. Two‐electron redox chemistry enables potassium‐free copper hexacyanoferrate as high‐capacity cathode for aqueous Mg‐ion battery. InfoMat 2024; 6: e12549. [Article] [Google Scholar]
- Liu Y, He B, Pu J, et al. High-energy fiber-shaped calcium-ion batteries enable integrated wearable electronics for human body monitoring. J Energy Chem 2024; 99: 661-670. [Article] [Google Scholar]
- Li H, Yang J, Cheng J, et al. Flexible aqueous ammonium-ion full cell with high rate capability and long cycle life. Nano Energy 2020; 68: 104369. [Article] [Google Scholar]
- Han L, Ling Y, Gong W, et al. In-situ dynamic compensation strategy enables jahn-teller effect suppression in Zn-doped dual-active-site copper hexacyanoferrate cathodes for high-energy and ultra-stable ammonium-ion storage. Angew Chem Int Ed 2025; 64: e202507427. [Article] [Google Scholar]
- Yang J, Chen J, Wang Z, et al. Recent advances and prospects of fiber-shaped rechargeable aqueous alkaline batteries. Adv Energy Sustain Res 2021; 2: 2100060. [Article] [Google Scholar]
- Mo F, Liang G, Huang Z, et al. An overview of fiber-shaped batteries with a focus on multifunctionality, scalability, and technical difficulties. Adv Mater 2020; 32: 1902151. [Article] [Google Scholar]
- Lu Y, Goodenough JB, Kim Y. Aqueous cathode for next-generation alkali-ion batteries. J Am Chem Soc 2011; 133: 5756-5759. [Article] [Google Scholar]
- Wang Y, Gao Y, He J, et al. Sphere‐confined reversible Zn deposition for stable alkaline aqueous batteries. Adv Mater 2024; 36: 2307819. [Article] [Google Scholar]
- Chen C, Tang Z, Li J, et al. MnO enabling highly efficient and stable Co‐Nx/C for oxygen reduction reaction in both acidic and alkaline media. Adv Funct Mater 2023; 33: 2210143. [Article] [Google Scholar]
- Li Y, Huang A, Zhou L, et al. Main-group element-boosted oxygen electrocatalysis of Cu-N-C sites for zinc-air battery with cycling over 5000 h. Nat Commun 2024; 15: 8365. [Article] [Google Scholar]
- Wang X, Ouyang T, Wang L, et al. Redox‐inert Fe3+ ions in octahedral sites of Co‐Fe spinel oxides with enhanced oxygen catalytic activity for rechargeable zinc-air batteries. Angew Chem 2019; 131: 13425-13430. [Article] [Google Scholar]
- Xu Y, Zhang Y, Guo Z, et al. Flexible, stretchable, and rechargeable fiber-shaped zinc-air battery based on cross-stacked carbon nanotube sheets. Angew Chem Int Ed 2015; 54: 15390-15394. [Article] [Google Scholar]
- Ma L, Chen S, Wang D, et al. Super‐stretchable zinc-air batteries based on an alkaline-tolerant dual-network hydrogel electrolyte. Adv Energy Mater 2019; 9: 1803046. [Article] [Google Scholar]
- Li M, Meng J, Li Q, et al. Finely crafted 3D electrodes for dendrite-free and high-performance flexible fiber-shaped Zn-Co batteries. Adv Funct Mater 2018; 28: 1802016. [Article] [Google Scholar]
- Zeng Y, Meng Y, Lai Z, et al. An ultrastable and high-performance flexible fiber-shaped Ni-Zn battery based on a Ni-NiO heterostructured nanosheet cathode. Adv Mater 2017; 29: 1702698. [Article] [Google Scholar]
- Sun Y, Natsuki J, Xu S, et al. High-performance flexible zinc-ion battery: Slurry-coated on carbon fiber. Mater Lett 2024; 371: 136866. [Article] [Google Scholar]
- Li C, Wang W, Tang Y, et al. Building microcracked structure fibrous cathode coated by poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) for ultra-stable fiber-shaped aqueous zinc ions batteries. J Colloid Interface Sci 2025; 677: 551-559. [Article] [Google Scholar]
- Kareri T, Hossain MS, Ram MK, et al. A flexible fiber‐shaped hybrid cell with a photoactive gel electrolyte for concurrent solar energy harvesting and charge storage. Intl J Energy Res 2022; 46: 17084-17095. [Article] [Google Scholar]
- Wang C, He T, Cheng J, et al. Bioinspired interface design of sewable, weavable, and washable fiber zinc batteries for wearable power textiles. Adv Funct Mater 2020; 30: 2004430. [Article] [Google Scholar]
- Ye L, Hong Y, Liao M, et al. Recent advances in flexible fiber-shaped metal-air batteries. Energy Storage Mater 2020; 28: 364-374. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Deng C, Li Y, Huang J. Building smarter aqueous batteries. Small Methods 2024; 8: 2300832. [Article] [Google Scholar]
- Guan Q, Li Y, Bi X, et al. Dendrite-free flexible fiber-shaped Zn battery with long cycle life in water and air. Adv Energy Mater 2019; 9: 1901434. [Article] [Google Scholar]
- Liao M, Wang J, Ye L, et al. A high-capacity aqueous zinc-ion battery fiber with air-recharging capability. J Mater Chem A 2021; 9: 6811-6818. [Article] [Google Scholar]
- Xiong T, Zhou X, Wang Y, et al. Photo-powered all-in-one energy harvesting and storage fibers towards low-carbon smart wearables. Energy Storage Mater 2024; 65: 103146. [Article] [Google Scholar]
- Zhang N, Huang F, Zhao S, et al. Photo-rechargeable fabrics as sustainable and robust power sources for wearable bioelectronics. Matter 2020; 2: 1260-1269. [Article] [Google Scholar]
- Zhang H, Xiong T, Zhou T, et al. Advanced fiber-shaped aqueous Zn ion battery integrated with strain sensor. ACS Appl Mater Interfaces 2022; 14: 41045-41052. [Article] [Google Scholar]
- Zhang Q, Li L, Li H, et al. Ultra-endurance coaxial-fiber stretchable sensing systems fully powered by sunlight. Nano Energy 2019; 60: 267-274. [Article] [Google Scholar]
- Chen L, Zhou J, Wang Y, et al. Flexible, stretchable, water-/fire-proof fiber-shaped Li‐CO2 batteries with high energy density. Adv Energy Mater 2023; 13: 2202933. [Article] [Google Scholar]
- Ma J, Cui Z, Du Y, et al. Wearable fiber-based supercapacitors enabled by additive-free aqueous MXene inks for self-powering healthcare sensors. Adv Fiber Mater 2022; 4: 1535-1544. [Article] [Google Scholar]
- Li Y, Guan Q, Cheng J, et al. Amorphous H0.82MoO3.26 cathodes based long cyclelife fiber-shaped Zn-ion battery for wearable sensors. Energy Storage Mater 2022; 49: 227-235. [Article] [Google Scholar]
- Pu J, Gao Y, Cao Q, et al. Vanadium metal‐organic framework‐derived multifunctional fibers for asymmetric supercapacitor, piezoresistive sensor, and electrochemical water splitting. SmartMat 2022; 3: 608-618. [Article] [Google Scholar]
All Tables
Comparative summary of development status and key challenges for different fiber-shaped aqueous battery systems
All Figures
 |
Figure 1 Overview of FABs. (A) Main progress in FABs. (B) Basic descriptors of common aqueous charge carriers: ionic weight (red), crystal/covalent radius (blue), and hydrated radius (green). (C) Normalized, qualitative radar comparison of each ion in terms of affordability, safety, stability, reversibility in aqueous media, and volumetric capacity. |
| In the text | |
 |
Figure 2 Schematic illustration of typical energy storage mechanisms in FABs. |
| In the text | |
 |
Figure 3 Schematic illustration of typical fabrication methods for fiber electrodes. (A) In situ growth. (B) Solution spinning. (C) Surface coating. (D) Thermal drawing. |
| In the text | |
 |
Figure 4 Schematic illustrations of typical device architectures for FABs. (A) Parallel structure. (B) Twisted structure. (C) Coaxial structure. (D) Crossing structure. |
| In the text | |
 |
Figure 5 Research progress of lithium-ion and sodium-ion batteries. (A) Schematic illustration of a simplified structure of the FALBs. (B) Energy and power densities of the FALBs compared with previous energy storage systems. (C) Energy textile woven with FALBs. (A–C) Reproduced with permission from [17]. Copyright©2016, Royal Society of Chemistry. (D) Schematic diagram of the high-voltage flexible aqueous fiber LIB based on synergistic dual co-solvents hybrid electrolyte. Reproduced with permission from [64]. Copyright©2024, Elsevier. (E) Schematic illustration of the structure of FASBs. (F) Galvanostatic charge-discharge curves of FASBs. (G) Capacity stability of FASBs. (E–G) Reproduced with permission from [65]. Copyright©2017, Elsevier. (H) Schematic and charge-discharge curves of FASBs. Reproduced with permission from [24]. Copyright©2019, The Author(s). |
| In the text | |
 |
Figure 6 Research progress of zinc-ion batteries. (A) The schematic illustration of the fiber battery based on Zn wire and MnO2/carbon fiber. (B) The discharge curves of fiber battery with Zn wire or Zn powder/carbon fiber as negative electrode. (C) Two fiber batteries connected in series continuously drive a commercial green LED when bent around human finger. (A–C) Reproduced with permission from [80]. Copyright©2013, Elsevier. (D) Schematic of fabrication and encapsulation of yarn ZIBs to power luminescent panel. Reproduced with permission from [81]. Copyright©2018, American Chemical Society. (E) Schematic illustration of quenched Ni-V2O5 NWs@CNT fiber electrode. (F) Galvanostatic charge-discharge (GCD) curves. (E, F) Reproduced with permission from [82]. Copyright©2012, Elsevier. (G) Diagram of synthesis procedure of a-Ca-V2O5/CNTF. (H) Comparison of rate performance. (G, H) Reproduced with permission from [83]. Copyright©2023, Wiley-VCH. (I) Schematic of flexible high-voltage coaxial of FAZBs. Reproduced with permission from [1]. Copyright©2019, American Chemical Society. (J) Schematic illustration of as-fabricated FAZIB based on dual-layer gel electrolyte with lysine. (K) Cyclic property and coulombic efficiency of as-assembled FAZIB. Inset: GCD splines at selected cycle. (J, K) Reproduced with permission from [27]. Copyright©2024, Wiley-VCH. |
| In the text | |
 |
Figure 7 Research progress of magnesium-ion, calcium-ion and aluminum-ion batteries. (A) GCD curves at 8 A g−1 of NiOOH/CNT@CC obtained in 1 M MgCl2 and 1 M KOH. (B) Schematic diagram of the as-assembled FAMIBs. (C) Optical photo and structural schematic of the self-powered photoelectric sensing fabric. (A–C) Reproduced with permission from [91]. Copyright©2024, The Author(s). (D) The comparison of GCD curves from three electrode. (E) Schematic diagram of the FAMIBs. (F) Capacity retention of FAMIBs under different bending angles. (D–F) Reproduced with permission from [92]. Copyright©2024, The Author(s). (G) Schematic illustration of the full configuration of FACIBs. (H) GCD profiles under different bending angles at a fixed length (2 cm) and bending radius (0.5 cm) (inset: photographs for the bending test). (G, H) Reproduced with permission from [93]. Copyright©2024, Elsevier. (I) GCD profiles at different bending angles of fiber aqueous Al-ion battery. Reproduced with permission from [25]. Copyright©2022, The Author(s). |
| In the text | |
 |
Figure 8 Research progress of FAABs. (A) Schematic illustration of the CF@urchin-like NH4V4O10. (B) CV curve of the FAABs. (A, B) Reproduced with permission from [94]. Copyright©2020, Elsevier. (C) Schematic illustration of in-situ dynamic compensation strategy. (D) Theoretical calculations of Cu–N bond length changes during ammoniation/de-ammoniation progresses in ZnCuHCF. (E) Cycling performance of the ZnCuHCF. (F) The GCD curves of ZnCuHCF. (C–F) Reproduced with permission from [95]. Copyright©2025, Wiley-VCH. |
| In the text | |
 |
Figure 9 Research progress of alkaline aqueous batteries. (A) Schematic illustration of the fabrication of the FZABs. (B) Schematic illustration to the structure of the aligned CNT in the FZABs. (C) GCD curves of a FZABs at current densities of 1 A g−1. (A–C) Reproduced with permission from [103]. Copyright©2015, John Wiley and Sons. (D) Schematic illustration of 500% stretchable. (E) Power density curves of fiber-shaped highly stretchable FZABs at fully released state and 500% tensile strain with PANa-cellulose hydrogel electrolyte. (D, E) Reproduced with permission from [104]. Copyright©2019, John Wiley and Sons. (F) Schematics of the all-solid-state fiber-shaped Zn-Co battery. (G) Ragone plot of the all-solid-state fiber-shaped Zn-Co battery based on the volume of the whole flexible battery. (F, G) Reproduced with permission from [105]. Copyright©2019, John Wiley and Sons. (H) Schematic diagram of the flexible quasi-solid-state fiber-shaped Ni-NiO//Zn battery. (I) Cycling performance of the quasi-solid-state fiber-shaped Ni-NiO//Zn battery at various current densities. (H, I) Reproduced with permission from [106]. Copyright©2017, John Wiley and Sons. (J) Schematics of the fiber-shaped NiCo-Fe battery. (K) GCD curves of the fiber-shaped NiCo-Fe battery. (J, K) Reproduced with permission from [22]. Copyright©2019, John Wiley and Sons. |
| In the text | |
 |
Figure 10 Research progress of FABs. (A) Schematic illustration of the fabrication steps of the Ny66-Ag/CNT/ZnO-NWs anode electrode and the final device structure. Reproduced with permission from [109]. Copyright©2022, The Author(s). (B) Schematic diagram of fabrication and encapsulation of FABs. Reproduced with permission from [81]. Copyright©2018, American Chemical Society. (C) The performances of sewn and woven power textiles based on the FABs. Reproduced with permission from [110]. Copyright©2020, John Wiley and Sons. (D) Schematic of the FABs with air-recharging capability integrated into multifunctional wearable systems. Reproduced with permission from [114]. Copyright©2021, Royal Society of chemistry. (E) Cycling performance with or without Mn2+ in the electrolyte of FABs. (F) The photo-rechargeable fabric delivered stable electrical output under varying environmental disturbance. (E, F) Reproduced with permission from [116]. Copyright©2020, Elsevier. (G) The performance of wearable sensor device integrated with FABs. Reproduced with permission from [121]. Copyright©2022, Elsevier. (H) I-T curves of the device at different incidence angles. Reproduced with permission from [85]. Copyright©2023, American Chemical Society. (I) Fluorescent display for FABs. Reproduced with permission from [84]. Copyright©2023, American Chemical Society. |
| In the text | |
 |
Figure 11 Challenges of large-scale industry production of FABs. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

